Cholinergic Degeneration and Cognitive Function in Early GBA1-Related Parkinson's Disease
Trial registration: NCT04180865.
Abstract
Objective
The phenotype of patients with Parkinson's disease carrying GBA1 variants (GBA-PD) suggest similarities to symptomatology associated with early cholinergic system degeneration. Therefore, this study aims to investigate the clinical features and the cholinergic innervation pattern in patients with early GBA-PD versus those without the GBA1 mutation (non-GBA-PD).
Methods
A total of 46 GBA-PD and 104 non-GBA-PD subjects were included. Clinical assessments included motor and non-motor evaluation, as well as a comprehensive neuropsychological examination. Cholinergic system integrity was assessed using 18F-Fluoroethoxybenzovesamicol (18F-FEOBV) positron emission tomography (PET) to investigate the differences between GBA-PD and non-GBA-PD. Given the higher prevalence of females in GBA-PD, analyses were repeated when stratified by sex. Additionally, we examined the association between cognitive domains and whole-brain cholinergic binding in both groups. Exploratory analyses examined clinical and 18F-FEOBV binding differences among GBA1 variants.
Results
GBA-PD patients exhibited a higher burden of non-motor symptoms and lower cognitive performance on executive functions and attention. We observed a more pronounced cholinergic denervation in GBA-PD, compared to non-GBA-PD, primarily in the anterior, central, and limbic regions. However, the distribution of cholinergic loss and its association with attention and executive dysfunction was comparable between GBA-PD and non-GBA-PD. In addition, the clinical presentation and cholinergic binding differed significantly between sexes.
Interpretation
These results suggest an important role of early cholinergic denervation in GBA-PD patients, which is related to more severe cognitive dysfunction. ANN NEUROL 2025;98:398–409
The GBA1-gene is responsible for encoding the lysosomal enzyme glucocerebrosidase and has an important role in Parkinson's disease (PD).1 GBA1 variant carriers have an increased risk for PD.2-6 In a biallelic state, GBA1 variants classified as mild or severe cause Gaucher's disease (GD), a rare lysosomal storage disorder.7 The E326K and T369M variants of GBA1, which do not cause GD, are known to serve as risk factors for PD.8-10
Parkinson's disease with a GBA1 variant (GBA-PD) is linked to a more severe disease course.11-13 The GBA-PD phenotype is especially associated with various non-motor symptoms.14 GBA-PD exhibits a higher frequency of mild cognitive impairment (MCI) and progression to dementia compared to non-GBA-PD.2, 15, 16 A previous study found lower performances in working memory, executive function, and visuospatial abilities in both pathological GD-associated variants and E326K carriers compared to non-GBA-PD.17 In addition, visual short-term memory deficits18 and nonverbal memory impairment15 are associated with N370S and L444P mutations of GBA-PD. Neuropsychiatric disturbances, including depression, anxiety, and hallucinations, are more prevalent in GBA-PD.11, 19, 20 Furthermore, GBA-PD is linked to postural instability and gait difficulties, rather than tremor-dominant features.8
The GBA-PD phenotype shares similarities with symptomatology associated with cholinergic system degeneration. We have previously demonstrated evidence of deficits in cholinergic innervation in de novo PD, both with and without GBA1 variants, using 18F-fluoroethoxybenzovesamicol (18F-FEOBV) positron emission tomography (PET).21 At the time of diagnosis, GBA-PD showed more extensive cholinergic denervation in the occipital, parietal, and temporal regions than non-GBA-PD, when compared to controls, with a similar cognitive performance.
The distinct phenotype of GBA-PD is anticipated to become more evident as the disease progresses. Therefore, we aim to expand on our previous findings in de novo patients by investigating the clinical symptomatology and cholinergic innervation in GBA-PD patients during more advanced disease stages (mean disease duration, ~4 years).
The goal of this study was to characterize the clinical features of early GBA-PD relative to non-GBA-PD patients and how these features relate to regional brain cholinergic innervation using extensive neuropsychological testing and 18F-FEOBV PET.
Methods
Settings and Participants
We collected data from 150 PD patients (46 GBA-PD and 104 non-GBA-PD). All PD subjects met the criteria for PD diagnosis according to the Movement Disorders Society (MDS) clinical diagnostic criteria for PD and were treated by a movement disorder specialist. The majority of the patients (90 non-GBA and 17 GBA-PD) were enrolled in the Dutch Parkinson Cohort (DUPARC) study.22 Twenty-six GBA1 carriers who participated in a nationwide GBA1 screening were recruited to additionally participate in the DUPARC study protocol. Other patients with similar disease duration (14 non-GBA-PD and 3 GBA-PD) were recruited as part of another ongoing study in our laboratory, which follows the same clinical and imaging protocols.23 All subjects underwent 18F-FEOBV PET imaging, a T1-weighted magnetic resonance imaging (MRI) scan, and a standardized clinical and neuropsychological examination. Exclusion criteria included the use of medication with anticholinergic effects (eg, clozapine),24 inability to provide written informed consent, an estimated low premorbid intelligence level (estimated intelligence quotient [IQ] <70 on the Dutch Adult Reading test),25 and MRI contra-indications (eg, ferrous objects in the body or claustrophobia). Subjects taking cholinesterase inhibitors (ChEI) were not excluded from this study, despite the potential influence on cognition. ChEIs are prescribed for patients with Parkinson's disease dementia (PDD), being part of our population. Given that the GBA1-related PD phenotype is associated with greater cognitive dysfunction, excluding participants taking ChEI would disproportionately impact the representation of GBA-PD cases. All subjects gave written informed consent, and the study was approved by the local ethics committee.
Genotyping
Saliva was obtained from PD patients using Oragene DNA OG-500 tubes (DNA Genotek). DNA isolation, next generation sequencing (NGS), and data analysis were performed by GenomeScan B.V., Leiden, the Netherlands. Primers were selected to unambiguously sequence the functional GBA1-gene and not the pseudogene, using long-range polymerase chain reaction (PCR). Fragments were amplified using PCR and sequenced using Illumina cBot and HiSeq 400 as described previously.26 The allelic nomenclature of GBA1 variants is provided. Accordingly, GBA1 variants were classified as risk, mild, severe, or unknown following the classifications presented in the GBA1-PD browser (https://pdgenetics.shinyapps.io/GBA1Browser/).The classification of mild and severe variants is based on the type of GD that they cause in the homozygous state. Risk variants do not cause GD, but serve as risk factors of PD.8-10
Clinical Assessment
Cognitive Assessment
All subjects underwent comprehensive neuropsychological assessment (see Data S1 for details). The 5 main cognitive domains were covered: memory, attention, executive functions, language, and visuospatial abilities. All (sub)test scores were transformed into standardized T-scores based on established test-specific normative data. T-scores within a cognitive domain were averaged to define a domain-specific T-score for each domain. Patients were tested in the “on” medication state.
Motor Performance
Clinical motor performance was examined using the MDS Unified Parkinson's Disease Rating Scale part III (MDS-UPDRS-III) in the dopaminergic “on” state. Specific items from the UPDRS II and III were used to classify motor phenotype (tremor-dominant, postural instability and gait difficulty, or indeterminate), based on standard criteria.27 The levodopa equivalent daily dose (LEDD) was calculated.28
Non-Motor Assessment
Presence of non-motor symptoms was assessed using the Hospital Anxiety and Depression Scale, Non-Motor Symptoms Questionnaire (NMS-Q), rapid eye movement (REM) Sleep Behavior Disorder Screening Questionnaire (RBD-Q), and Burghart Sniffin’ Sticks 12 test.22 Higher scores on these questionnaires indicate more symptoms/complaints. Participants completed the questionnaires at home.
Brain Image Acquisition and Pre-Processing of 18F-FEOBV PET Imaging
All participants underwent brain resonance imaging (MRI) and vesicular acetylcholine transporter (VAChT) PET imaging using 18F-FEOBV radiotracer while in the “on” medication state. Acquisition procedures are detailed in the supplementary materials S2. The 18F-FEOBV PET data was pre-processed using a previously published pipeline.29 Statistical parametric mapping (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) software was used to realign the PET imaging frames within subjects to reduce the effect of subject motion during imaging sessions. PET-MRI registration was performed using T1-weighted MRI volumetric scans. Freesurfer software (Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA) was used to run the recon-all function (default setting) for all MRI images. Parametric images reflecting distribution volume ratios (DVR) were constructed of 18F-FEOBV in the brain using supratentorial white matter above the ventricles (morphologically eroded) as a reference region.29 Müller-Gärtner partial-volume correction (PVC) approach was used on the parametric PET images. Following recently published guidelines, we conducted our analyses with and without PVC to verify any variation of the results.30
Voxel-Wise Analyses Pre-Processing
Parametric images with and without PVC were normalized to a study-specific template in Montreal Neurological Institute (MNI) space using high-dimensional DARTEL registration. We applied an 8mm full width at half maximum smoothing filter to improve the signal-to-noise ratio.
Volumes of Interest-Based Analyses Pre-Processing
Cortical and subcortical labels from Deskian/Killiany and Aseg atlas parcellations were used to obtain grey matter subject-specific volumes of interest (VOIs). We used 33 cortical-cerebellar (Desikan/Killiany atlas) and 8 sub-cortical VOIs (Aseg atlas). We extracted the mean DVR values from 18F-FEOBV PET parametric images with and without PVC in native space using the 41 MRI-derived VOIs.
Imaging Analyses
GBA-PD Versus Non-GBA-PD
We performed a voxel-wise SPM12 2 sample t test to compare GBA-PD to non-GBA-PD. The analysis was performed with and without the possibly confounding variables disease duration and LEDD. Because the 2 groups differed in sex distribution, we also performed complementary voxel-wise analyses comparing female GBA-PD to female non-GBA-PD and male GBA-PD to male non-GBA-PD. Previous studies have shown that male PD patients have more severe cholinergic denervation compared to female patients.31 Given that our non-GBA group had a higher prevalence of males, we wanted to check if this would lead to an underestimation of the differences between GBA1 and non-GBA1 patients. We decided to stratify the analysis by sex rather than use it as a covariate because previous studies found a higher prevalence of females in GBA1-associated PD, and therefore, it might be an unbalanced factor. Voxel-wise analyses were done to compare subjects taking ChEIs versus patients not taking this medication, using t tests. In addition, we used the Dice similarity coefficient,32 to quantify the degree of overlap between the t-maps resulting from each 2-sample t test SPM model. This metric measures volume overlaps between 2 regions divided by their mean volume. It is interpreted as follows: <0.2, poor; 0.2 to 0.4, fair; 0.4 to 0.6, moderate; 0.6 to 0.8, good; and >0.8, excellent agreement.33
Cognitive Symptoms and Cholinergic Association
Voxel-wise multiple regression analyses were performed to examine both positive and negative correlations between cognitive domain T-scores and whole-brain cholinergic binding topography in GBA-PD and non-GBA-PD patients. Our aim was to investigate whether GBA-PD and non-GBA-PD had different patterns of cholinergic denervation, related to the cognitive domain scores.
Explorative Analysis: GBA-PD Variants
As an exploratory analysis we assessed the different GBA1 variants, distinguishing GBA-PD patients with a risk variant from those with mild or severe variants. Three groups (1) GBA1 risk variants, (2) GBA1 mild and severe variants, and (3) non-GBA-PD were compared based on the mean uptake values extracted from the mask derived from the significant results between GBA-PD and non-GBA-PD. We merged the mild and severe variants to equalize the sample sizes of the GBA-PD groups.
All voxel-wise analyses were implemented in SPM12. Voxel-wise results were first explored with a voxel-level threshold of p < 0.05, false discovery rate (FDR) corrected, cluster extent (K) >50. If no significant clusters appeared, we explored an uncorrected threshold of p < 0.005.
Statistical Analyses
We evaluated the differences in demographic, clinical variables using an independent sample t test or Mann–Whitney U test, for normally and non-normally distributed data, respectively. Differences between PD GBA1 variants were tested with an analysis of variance test for continuous variables and χ2 testing for dichotomous variables. Correlations between DVR VOI values with and without PVC were calculated using Spearman's rank correlation or Pearson correlation according to the distribution of the variables. The statistical threshold was set at p < 0.05. Statistical analyses were performed using SPSS (version 28.0) and RStudio (version 4.3.2).
Results
GBA1 Genetic Screening
In the GBA-PD group of 46 subjects, 13 different GBA1 nonsynonymous variants were identified (S3). We found 26 subjects with a risk variant, 11 subjects with a mild variant, and 6 subjects with severe variants. Additionally, 3 subjects exhibited a variant that is classified as having an unknown severity.34
Participant Demographics and Clinical Assessments
The 150 PD patients had a mean (standard deviation [SD]) age of 66.7 (8.6) years and a mean (SD) disease duration of 3.8 (1.8) years. Demographic and clinical data of the GBA-PD and non-GBA-PD groups are presented in Table 1. The GBA-PD group contained a significantly higher percentage of females compared to non-GBA-PD and exhibited a higher NMS-Q total score. GBA-PD had a significantly lower mean T-score on attention and executive functioning, reflecting worse performance.
| GBA-PD (n = 46) | non-GBA-PD (n = 104) | p | |
|---|---|---|---|
| Age, yr, mean (SD) | 68.1 (8.4) | 66.4 (8.7) | 0.162 |
| Gender, M (% M) | 27 (58.7) | 82 (78.8) | 0.019 |
| Disease duration, yr | 4.2 (2.0) | 3.6 (1.7) | 0.072 |
| Educational level (mean [Q1–Q3]) | 5.00 [4.00–6.00] | 5.00 [4.25–6.00] | 0.329 |
| Use of cholinesterase inhibitor, n (%) | 5 (10.9) | 3 (2.9) | 0.110 |
| Motor symptoms | |||
| MDS-UPDRS-III | 28.00 [20.00–42.00] | 27.00 [17.00–34.00]a | 0.236 |
| Hoehn and Yahr stage (%) | 2.00 [2.00–3.00] | 2.00 [2.00–2.00] | 0.065 |
| LEDD | 700.00 [425.00–956.50] | 600.00 [450.00–961.00] | 0.363 |
| Motor phenotype, n TD/PIGD/indeterminate | 17 (37.0%)/ 20 (45.5%)/ 7 (15.9%)b | 39 (45.3%)/ 37 (43.0%)/ 10 (11.6%)c | 0.251 |
| MDS-UPDRS-II | 13.64 (8.2)b | 10.94 (6.0)d | 0.057 |
| Non-motor symptoms | |||
| MoCA, total score | 26.00 [23.00–27.50] | 26.00 [23.00–28.00] | 0.369 |
| NMS-Quest, total score | 9.00 [5.00–12.00]e | 6.00 [4.00–10.00]f | 0.016 |
| HADS anxiety, total score | 4.73 (3.5)g | 3.83 (3.0)h | 0.154 |
| HADS depression, total score | 4.80 (3.4)g | 3.99 (3.1)h | 0.196 |
| RBD Quest, total score | 5.62 (3.7)a | 4.65 (3.2)b | 0.128 |
| Sniffin’ sticks, total score | 5.87 (2.4) | 5.35 (2.2) | 0.211 |
| Cognition domain scores | |||
| T-score memory | 45.65 (7.5) | 47.45 (8.4) | 0.331 |
| T-score attention | 39.72 (9.1) | 43.22 (9.5)b | 0.036 |
| T-score executive function | 42.41 (9.9) | 46.58 (9.4) | 0.018 |
| T-score visuospatial function | 42.72 (13.3) | 45.37 (12.4) | 0.255 |
| T-score language | 46.32 (10.2)b | 49.69 (9.2)h | 0.069 |
- a Missing, n = 1.
- b Missing, n = 2.
- c Missing, n = 18.
- d Missing, n = 15.
- e Missing, n = 3.
- f Missing, n = 19.
- g Missing, n = 5.
- h Missing, n = 16.
- Abbreviations: Educational level = according to the Dutch Verhage scale; HADS = Anxiety and Depression Scale; LEDD = levodopa equivalent daily dose; MDS-UPDRS-II and III = Movement Disorders Society Unified Parkinson's Disease Rating Scale part II and III; MoCA = Montreal Cognitive Assessment; NMS-Quest = Non-Motor Symptoms Questionnaire;PIGD = Postural Instability and Gait Difficulties; TD = Tremor Dominant; RBD Quest = NMS-REM Sleep Behavior Disorder Screening Questionnaire; Sniffin’ sticks = Burghart Sniffin’ Sticks 12 Test.
Cholinergic Comparisons
GBA-PD Versus Non-GBA-PD
GBA-PD exhibited lower 18F-FEOBV binding than non-GBA-PD in various bilateral frontal regions, including inferior, medial, middle, and superior frontal gyri, as well as the precentral, paracentral, and postcentral gyri. Lower binding was also observed in the lingual gyri, precuneus, thalami—particularly the ventral lateral nucleus—and limbic structures, such as the insula, cingulate gyrus, and parahippocampal gyri. Additionally, the middle and inferior occipital gyrus, along with the angular gyrus and cuneus in the left hemisphere, showed lower VAChT binding in GBA1 carriers versus non-carriers (Fig 1). The data showed no significant voxels in opposite direction (18F-FEOBV binding GBA-PD > non-GBA-PD).
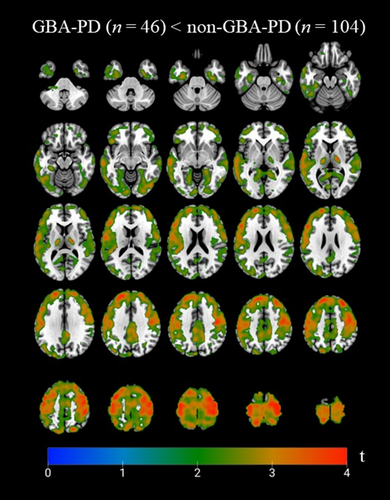
Partial volume correction with the Müller-Gärtner method did not change the voxel-wise results in any cortical region (S4). Additionally, bivariate correlation analyses of the DVRs of the subcortical volume-of-interest showed strong correlations between regions with and without partial volume correction, except for the pallidum (S5). Therefore, we decided to present all data without additional partial volume correction.
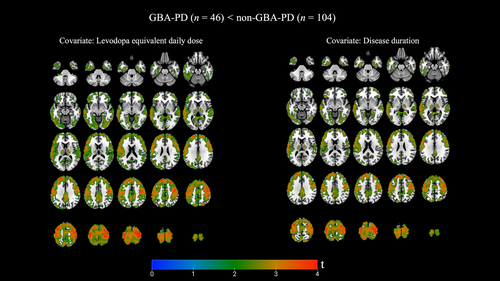
GBA-PD Versus Non-GBA-PD: Covariate Disease Duration and LEDD
Although not statistically significant, clinical characteristics comparing both PD groups demonstrated a slightly longer disease duration in GBA-PD (mean [SD] 4.2 [2.0] years) compared to non-GBA-PD (mean [SD] 3.6 [1.7] years). In addition, patients were tested in the “on” dopaminergic medication state. Bidirectional whole brain voxel-based analyses were therefore repeated, while controlling for both disease duration and LEDD. As for the 2 maps, GBA-PD exhibited lower 18F-FEOBV binding in comparable pattern compared to non-GBA-PD, involving the same brain regions (Fig 2). Reverse directions again showed no results.
Post Hoc: Stratification by Sex
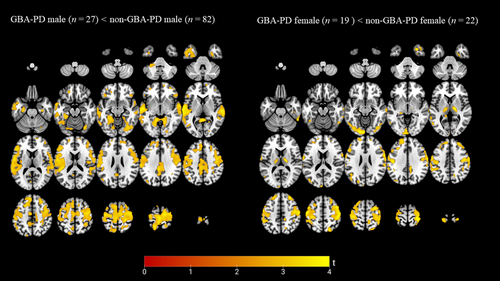
The GBA-PD group had a significantly higher percentage of females compared to non-GBA-PD group. GBA-PD males presented with significantly lower performance on the cognitive domains attention, executive functions, and language compared to non-GBA-PD males. A significantly higher score on the MDS-UPDRS II, NMS-Q, and RBD questionnaire was found in GBA-PD females compared to non-GBA-PD females. See Supporting Information S1 for results of demographics and clinical characteristics when stratified by sex (S6 and S7).
Voxel-wise comparison did not reveal any significant lower VAChT binding in GBA-PD females versus non-GBA-PD females, using p < 0.05 FDR corrected at the voxel level. Therefore, we lowered the threshold to an uncorrected p < 0.005, resulting in a lower binding in the left > right inferior and middle occipital gyrus, left lingual gyrus, and bilateral precentral, and postcentral gyri, superior frontal gyri, and thalamus in female GBA-PD. We also found a lower VAChT binding in GBA-PD male versus non-GBA-PD male in the cingulate gyrus (anterior and posterior), both lingual gyri, parahippocampal gyri, superior and transverse temporal gyri, precentral and postcentral gyri, and medial and superior frontal gyri showed lower VAChT binding in GBA-PD. See Figure 3 for voxel-wise results stratified by sex. No significant results were observed in the reserve direction. The Dice coefficient showed an overlap of 0.2514, comparing the lower cholinergic binding patterns between female; (GBA-PD female vs non-GBA-PD female) versus male; (GBA-PD male vs non-GBA-PD male), indicating a very limited overlap in these distributions.
Correlations of Cognitive Performance with Voxel-Based Regional 18F-FEOBV Binding
Whole-brain voxel-based analyses were performed to explore the correlation between regional brain VAChT binding and T-scores on attention and executive functioning, which were statistically significantly different between GBA-PD and non-GBA-PD. Only positive correlations were observed, indicating that lower cholinergic binding was associated with worse cognitive performance. The t-values of the voxel-wise analyses represent the strength of these correlations in the respective brain regions.
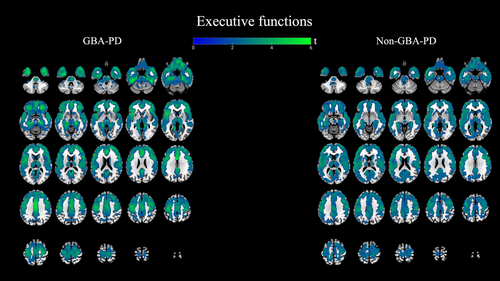
Executive Functions
We found widespread regional cerebral cholinergic correlations in both GBA-PD and non-GBA-PD. Both correlation analyses showed the involvement of the bilateral frontal and temporal cortex, parahippocampal gyri, lingual gyri, anterior and posterior cingulate gyrus, insula, thalami, lentiform nucleus, caudate, and pons. See Figure 4.
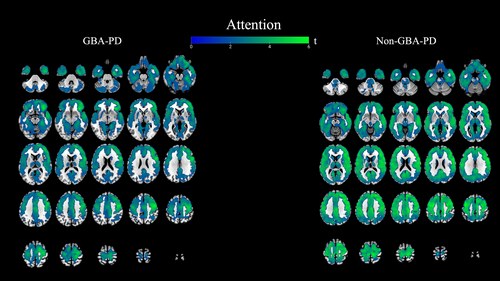
Attention
In the GBA-PD group, attentional performance showed widespread correlations with 18F-FEOBV binding in the frontotemporal regions (right precentral, inferior and middle frontal gyrus, cingulate gyrus, both parahippocampal gyri, temporal lobes, and uncus) and the posterior lobe of the cerebellum. A similar, yet more widespread, pattern was observed in non-GBA-PD, involving all cortical areas except the cerebellum. In the posterior cingulate, (pre)cuneus, and the thalami, correlations were more pronounced in non-GBA-PD versus GBA-PD. See Figure 5.
To assess the possible impact of ChEI, we calculated the overlap between the correlation maps of the complete GBA-PD group versus GBA-PD group excluding subjects taking ChEI, showing an overlap of, respectively, 0.8360 for executive function and 0.7545 for the attention domain. Additionally, we analyzed cognitive performance and regional 18F-FEOBV uptake between subjects taking ChEI to those without, regardless of their GBA1 status. The participants on ChEI (n = 8) did not differ from those not taking the medication (n = 142) in terms of age, disease duration, or educational level. However, subjects taking ChEI performed significantly worse across the attention, executive function, and memory domains (S8). Additionally, regional 18F-FEOBV uptake in ChEI users was lower in nearly all cortical areas, except for parts of the right > left occipital and temporal lobes. Lower VAChT binding was also observed in limbic regions, including the cingulate gyrus, insula, and parahippocampal gyrus, and the thalamus of ChEI users compared to those not taking the medication (S9).
Explorative Analysis: Genotype–Phenotype Correlations of Risk, Mild, and Severe GBA Variants
GBA-PD mild and severe variants exhibited a significant lower T-scores on the domain memory compared to GBA1 patients carrying a risk variant (S10). The mean 18F-FEOBV uptake values extracted from the mask derived from the significant results between GBA-PD and non-GBA-PD revealed a significant lower 18F-FEOBV binding when GBA1 risk variants (n = 26) and GBA1 mild and severe variants (n = 17) were compared to non-GBA-PD (n = 104). Differences between both groups of GBA1 variants were found (S11).
Discussion
This study investigated the clinical features and brain cholinergic denervation patterns of early GBA-PD. Our previous work showed that, at the time of diagnosis, GBA-PD exhibited more extensive posterior cholinergic denervation than non-GBA-PD compared to controls. This finding was not associated with clinical differences between both PD groups. However, this study, which included early-stage PD patients, demonstrated a higher overall burden of non-motor symptoms, including lower cognitive performance in executive function and attention in the GBA-PD group-consistent with other studies on early GBA-PD.11, 35 No significant differences between groups were found for specific non-motor item questionnaires, such as RBD, hyposmia, anxiety, and depression. Additionally, we observed more pronounced cholinergic denervation in early GBA-PD compared to non-GBA-PD. However, in contrast to de novo PD, the pattern of degeneration was now primarily observed in the anterior and central cortical areas, as well as in the limbic regions.
Clinical Phenotype
In this study, 56.5% of GBA-PD patients carried a risk variant and not a GD-related mutation, which is typically associated with a milder phenotype. These are the most common GBA1 variants in the Netherlands.26 This might explain why we did not find a higher burden on most specific non-motor items. Additionally, previous data reported that GBA-PD and non-GBA-PD were clinically indistinguishable at the time of diagnosis.2, 36, 37 Further phenotypical distinction between GBA1 carriers and non-GBA carriers might become more apparent during later disease stages.36, 38
Few studies have attempted to characterize the cognitive profile of GBA-PD using a complete cognitive test battery. One study found lower performance in working memory, executive function, and visuospatial domain in both GD associated GBA1 variants and E326K carriers compared to non-GBA-PD.17 Another study compared young-onset PD patients with L444P or N370S variants to patients without these variants and found significantly worse performance in the memory and visuospatial domains.15 The mean disease duration of patients in both studies was considerably longer, respectively, 15.4 and ~13 years compared to 3.8 years in our study. Previous longitudinal studies have reported diverging rates of cognitive deterioration in GBA-PD patients at ~3 years post-diagnosis.2, 36, 38 Our results suggest a profile of early cognitive decline in GBA-PD patients. The general progression of cognitive deficits in PD usually start with impairments in attention and executive function.39
Cholinergic Denervation Pattern
A decrease in 18F-FEOBV binding was observed in GBA-PD versus non-GBA-PD, particularly in bilateral frontal regions, central areas, and limbic structures. Previous cross-sectional PET imaging data suggest a denervation gradient over time, progressing from posterior-to-anterior cortices, accompanied by cognitive worsening.40, 41 A recent PET study examining longitudinal changes in cortical cholinergic activity in PD found most severe denervation in posterior regions at baseline, with a posterior-to-anterior gradient and involvement of anteriorly located regions (in an anterior-to-posterior pattern) over time.42 The greatest regions of denervation between patients with de novo dementia with Lewy bodies (DLB) and PD patients 8 years post-diagnosis has been observed in more anterior regions of the brain, including the more anteriorly located regions, including the limbic network. The authors therefore proposed that the cholinergic changes in PD evolve over time and become similar to those in PDD and DLB.43 Our current findings similarly demonstrate more severe cholinergic loss in anterior located and limbic regions in GBA-PD compared to non-GBA-PD, suggesting a more advanced pattern of cholinergic degeneration in GBA1 carriers, already present in early disease stages.
Our data on de novo PD demonstrated predominantly posterior cholinergic loss compared to controls in both GBA-PD and non-GBA-PD, consistent with previously reported findings.21, 40, 44-47 In addition, prior research has shown that variable posterior cholinergic denervation is also present in non-demented PD.48 This indicates that the clinical onset of cognitive impairment because of cholinergic changes in PD may depend more on a widespread network dysfunction rather than localized cortical changes.46, 49 It has even been proposed that cholinergic innervation of posterior subregions may be affected already before PD diagnosis.47 Therefore, the absence of significant differences in posterior cholinergic binding in the comparison between GBA-PD and non-GBA-PD may reflect a similar degree of cholinergic loss in these regions in both groups, resulting in no detectable differences.
GBA-PD performed significantly worse in the cognitive domains of executive function and attention. To gain a clearer understanding of the cholinergic system's role in the observed clinical differences between GBA-PD and non-GBA-PD, we conducted voxel-based correlation analyses. These correlation maps visualized cholinergic brain regions associated with cognitive domain performance in GBA-PD and non-GBA-PD separately. For executive function, 18F-FEOBV binding correlation maps revealed that the same brain regions were involved in both GBA-PD and non-GBA-PD patients, indicating that executive impairments may arise from similar mechanisms in PD, regardless of the presence of the GBA1 mutation. Notably, these regions were more severely affected in our GBA-PD cohort, which also exhibited greater executive deficits. The same holds true for attention. Widespread cerebral cholinergic denervation areas were found in both GBA-PD and non-GBA-PD. The extensive topography in both groups confirms widely distributed cholinergic involvement in attentional function.50 However, in non-GBA-PD, we found an even more pronounced regional cholinergic topography correlated with attention, possibly because of the larger sample size of non-GBA-PD patients, allowing more statistical power.
Evidence on sex distribution in GBA-associated PD is inconsistent across studies. However, a meta-analysis suggested a higher prevalence of females with GBA-PD in Europe and Northern America in GBA-PD.51 In line with this, we found a higher percentage of females in GBA-PD compared to non-GBA-PD. To investigate potential differences in clinical presentation and underlying cholinergic pathologies, we repeated the analysis stratified by sex. Both GBA-PD females and males exhibited lower cholinergic VAChT binding compared to their non-GBA-PD counterparts. Interestingly, we found notable differences in the overlap between both maps. This indicates a distinct cholinergic profile in GBA1-male and GBA1-female compared to sex-stratified non-GBA-PD. We propose that future research investigating the clinical features of GBA-PD and their underlying processes should therefore also concentrate on sex-specific effects.
Genotype–phenotype studies have shown that PD patients with severe or mild GBA1 variants present with more frequent motor and non-motor symptoms.11, 12 However, our exploratory analyses revealed no significant differences in 18F-FEOBV uptake between mild, severe, and risk variants of GBA-PD. Given the small sample sizes in this subgroup analysis, potential differences may have been missed, emphasizing the need for further investigation with well-powered studies.
Finally, future studies should also investigate GBA1 variants in non-PD individuals, as this population is essential for properly analyzing potential prodromal PD subjects.
Strengths and Limitations
Strengths of this study include the large sample size of PD patients with and without GBA1 mutations and an extensive and detailed subject assessment. The assessment comprised a clinical evaluation, the use of 18F-FEOBV PET, and a comprehensive neuropsychological test battery, using T-scores for the evaluation of cognitive functioning. The T-scores were already adjusted for age, gender, and educational level, eliminating these variables as possible by recovering components. Another strength is the use of full-gene GBA1 sequencing. A limitation of the study is that motor assessments were performed in the dopaminergic “on” state. Another limitation is the relatively small sample size of the subgroups of GBA1 variants. We did not exclude subjects taking ChEIs despite the possible positive impact on cognition, which might be considered as a limitation. However, excluding participants taking ChEI would have disproportionately impact the representation of GBA-PD cases, introducing selection bias. Notably, patients on ChEIs still performed statistical significantly worse in the executive function, attention, and memory domain, along with lower regional 18F-FEOBV uptake compared to the patients not taking ChEIs. Furthermore, the correlation maps between 18F-FEOBV uptake and cognition did not differ between the overall GBA-PD group versus the GBA-PD group without the subjects taking ChEIs. Last, 18F-FEOBV is a selective presynaptic tracer, and there is no proven effect of rivastigmine on the tracer signal.43
Conclusions
Overall, our findings suggest that cholinergic denervation relates to poor cognitive performance, regardless of the presence of a GBA1 variant. However, GBA-PD patients in our cohort presented with worse cognitive functions and a more severe cholinergic deficit compared to non-GBA-PD. These deficits were particularly present in the fronto-temporal cortical regions and limbic structures. These results support the idea that GBA-PD may develop more severe cholinergic deficits earlier in the disease process, contributing to greater cognitive vulnerability. Longitudinal studies are needed to verify this hypothesis, taking into account also the sex-specific effects, as suggested by our data.
Acknowledgements
We thank all patients, caregivers, health-care professionals, and students who have contributed to and collaborated in this project. The inclusion of patients was established with help of the collaborative Parkinson Platform Northern Netherlands.
Author Contributions
Sofie Slingerland: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; visualization; writing – original draft. Sygrid van der Zee: Conceptualization; data curation; formal analysis; funding acquisition; methodology; supervision; writing – review and editing. Giulia Carli: Methodology; software; supervision; writing – review and editing. Anne C. Slomp: Data curation; methodology; project administration; writing – review and editing. Emile d'Angremont: Data curation; project administration; writing – review and editing. Jeffrey M. Boertien: Conceptualization; funding acquisition; methodology; writing – review and editing. Teus van Laar: Conceptualization; funding acquisition; methodology; supervision; writing – review and editing.
Potential Conflicts of Interests
Nothing to report.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.




