Neuroimaging Biomarkers for Friedreich Ataxia: A Cross-Sectional Analysis of the TRACK-FA Study
Abstract
Objective
We aimed to quantify differences in the brain and spinal cord between Friedreich ataxia and controls, stratified by age and disease stage, including for the first time in young children.
Methods
TRACK-FA is the largest prospective, longitudinal, multi-modal neuroimaging study in Friedreich ataxia to date. We assessed individuals with Friedreich ataxia and controls, 5 to 42 years, at 7 sites across 4 continents. The 17 imaging primary outcome measures (POMs) were selected from metrics that showed a significant longitudinal change in previous small-scale studies. These included brain and spinal cord morphometry (structural magnetic resonance imaging [MRI]) and microstructure (diffusion MRI); brain iron levels (quantitative susceptibility mapping); and spinal cord biochemistry (magnetic resonance spectroscopy). This study is registered with ClinicalTrials.gov (NCT04349514).
Results
Between February 2021 and August 2023, we assessed 169 individuals with Friedreich ataxia and 95 controls. Compared to controls, individuals with Friedreich ataxia had lower volume of dentate nucleus and superior cerebellar peduncles; smaller cross-sectional area of spinal cord; lower fractional anisotropy and higher diffusivity in spinal cord and superior cerebellar peduncles; and lower total N-acetyl-aspartate/myo-inositol ratio in spinal cord. Morphometric differences in spinal cord and superior cerebellar peduncles increased dramatically with age during childhood, with rapid development in controls, but not in Friedreich ataxia. Many imaging POMs showed significant associations with clinical severity.
Interpretation
Our findings provide strong imaging evidence of impaired development of spinal cord and superior cerebellar peduncles during childhood in Friedreich ataxia and open the way for the use of neuroimaging biomarkers in clinical trials. ANN NEUROL 2025;98:386–397
Friedreich ataxia is a rare, autosomal recessive neurodegenerative disease. A homozygous expansion of an intronic GAA trinucleotide repeat is present in the majority of those affected.1 Average age of onset is between 8 and 14 years, and the hallmark neurological features include gait and limb ataxia, and dysarthria.1 Natural history studies demonstrate highly differential progression across subpopulations stratified by age, genetic severity, disease stage, and duration.2, 3
Interventional trials for Friedreich ataxia often use clinical scales as outcome measures4, 5; however, these have limited sensitivity, necessitating longer trial durations and increased participants to obtain adequate statistical power. Additionally, clinical scales have shown limitations in certain populations, such as young pediatric or late-stage disesase,4, 6 resulting in the requirement to extrapolate effects. The recent approval of omaveloxolone, the first treatment for individuals with Friedreich ataxia (age 16 years and older), underscores the need for sensitive and clinically meaningful outcome measures.7
Neuroimaging biomarkers offer a promising avenue to overcome limitations associated with clinical scales.8 Small-scale neuroimaging studies in Friedreich ataxia have shown atrophy and microstructural changes in the cerebellum, cerebellar pathways, and spinal cord.9-13 However, modest sample sizes in single-site studies preclude stratification by age or disease severity. Few studies include children and none under 10 years old. Collaborative efforts have addressed some limitations, by meta-analyzing existing neuroimaging datasets, confirming that Friedreich ataxia is associated with regionally specific patterns of atrophy, the magnitude of which correlate with disease severity.14, 15 However, large-scale neuroimaging studies are urgently needed to validate sensitive biomarkers of disease progression.8
TRACK-FA16 is the largest prospective, longitudinal, multi-site, multi-modal neuroimaging study in Friedreich ataxia to date. Designed with principles similar to a clinical trial, TRACK-FA uses a harmonized protocol and rigorous quality control. It is the first multi-modal neuroimaging study to include individuals with Friedreich ataxia and matched controls spanning young children to adults. The cohort will be assessed at 3 timepoints (baseline, 12, and 24 months). The primary objective is to create a robust longitudinal dataset that will identify the most sensitive neuroimaging biomarker/s of disease progression, laying the groundwork for their integration into future clinical trials.
Here, we present cross-sectional data from the TRACK-FA cohort at baseline, focusing on neuroimaging primary outcome measures (POMs), to identify differences between Friedreich ataxia and control groups. Additionally, we stratified the Friedreich ataxia cohort by age and disease stage to explore their associations with the POMs.
Materials and Methods
Design and Participants
Baseline enrolment and testing was completed over 30 months between February 2021 and August 2023 (exceeding the originally planned 18-month recruitment period because of the coronavirus pandemic). Individuals with genetically confirmed Friedreich ataxia, who met inclusion criteria (age of disease onset ≤25 years; disease duration ≤25 years; Functional Staging for Ataxia Scale [FSS]17 score ≤5; modified Friedreich Ataxia Rating Scale [mFARS]18 score ≤65; absence of other neurological conditions), and matched controls (absence of diagnosed psychiatric or neurological conditions), were recruited from multiple international sites (Table 1). For both groups, a further inclusion criterion was ≥5 years of age at enrolment; exclusion criteria were magnetic resonance imaging (MRI) contraindications, metallic dental braces, and pregnancy. The recruitment target was ~300 participants (200 with Friedreich ataxia and ~100 controls). Controls were recruited based on age (±2 years), sex, handedness as measured by the Edinburgh Handedness Inventory,19 and years of education (±2 years). The Friedreich ataxia:control matching ratio was 2:1 for participants age ≥11 years and 1:1 for 5 to 10 years of age. These ratios were selected because rapid nervous system development in young children requires more precise matching, and because the number of children age 5 to 10 years was expected to be relatively small (~10% of total enrolment). The published TRACK-FA protocol reports on ethics committees, approval numbers, and dates.16 Participants or their surrogates provided written informed consent before enrolment.
| Recruitment age category (years) | Control | Friedreich ataxia | Total | ||||
|---|---|---|---|---|---|---|---|
| 5–10 | 11–17 | ≥18 | 5–10 | 11–17 | ≥18 | ||
| No. | 16 (10 F) | 29 (13 F) | 50 (25 F) | 20 (11 F) | 53 (23 F) | 96 (47 F) | 264 (129 F) |
| Age | 8.8 (1.5) | 14.4 (1.9) | 28.3 (6.6) | 8.5 (1.6) | 14.9 (1.7) | 28.3 (7.2) | 21.7 (9.5) |
| Education yr | 2.2 (1.3) | 7.7 (2.0) | 16.9 (2.9) | 2.0 (1.7) | 8.1 (1.9) | 15.3 (2.6) | 11.4 (5.3) |
| Shorter allele: no. of FXN GAA repeats (GAA1) | – | – | – | 845.5 (166.2)b | 737.5 (179.5)c | 567.2 (193.9)d | 654.3 (213.0) |
| Longer allele: no. of FXN GAA repeats (GAA2) | – | – | – | 1,014.7 (336.6)e | 845.5 (166.2) | 861.7 (244.8)f | 903.1 (239.5) |
| Age of onset | – | – | – | 4.5 (1.8) | 7.8 (3.0) | 15.0 (5.3) | 11.5 (6.0) |
| Disease duration | – | – | – | 4.0 (2.1) | 7.0 (3.3) | 13.3 (5.4) | 10.2 (5.8) |
| Clinical and functional measures | |||||||
| FSS 0–2, no. (%) | – | – | – | 15 (75) | 25 (47) | 22 (23) | 62 (37) |
| FSS 2.5–4, no. (%) | – | – | – | 4 (20) | 18 (34) | 41 (43) | 63 (37) |
| FSS 4.5–5, no. (%) | – | – | – | 1 (5) | 10 (19) | 33 (34) | 44 (26) |
| FSS, mean (SD) | – | – | – | 2.0 (0.9) | 2.7 (1.4) | 3.5 (1.2) | 3.1 (1.4) |
| mFARS | – | – | – | 36.6 (13.1) | 40.6 (13.2) | 43.8 (12.5) | 41.9 (12.9) |
| SARA | – | – | – | 9.8 (4.6) | 12.9 (6.7) | 15.9 (6.5)g | 14.2 (6.7) |
| ADL | – | – | – | 9.0 (5.4) | 10.7 (5.2) | 14.0 (5.7) | 12.4 (5.8) |
| Sitea | |||||||
| Melbourne, Australia | 3 | 5 | 7 | 1 | 7 | 16 | 39 (14.8%) |
| Aachen, Germany | 5 | 10 | 9 | 8 | 12 | 23 | 67 (25.4%) |
| Minneapolis, USA | 3 | 6 | 5 | 4 | 8 | 7 | 33 (12.5%) |
| Gainesville, USA | 0 | 1 | 12 | 0 | 5 | 18 | 36 (13.6%) |
| Philadelphia, USA | 5 | 7 | 11 | 7 | 21 | 25 | 76 (28.8%) |
| Montréal, Canada | 0 | 0 | 6 | 0 | 0 | 7 | 13 (4.9%) |
- Note: Data are mean (SD) or no. (%).
- a Sites in Melbourne are Monash University and Murdoch Children's Research Institute; in Aachen is Rheinisch-Westfälische Technische Hochschule Aachen (RWTH Aachen University); in Minneapolis is University of Minnesota; in Gainesville is University of Florida; in Philadelphia is Children's Hospital of Philadelphia; in Montréal is McGill University. Site in Campinas, Brazil (The University of Campinas) is omitted because of unforeseen upgrade-related MRI scanner issues at that site.
- b GAA1 repeat length data was missing for one participant in the 5- to 10-year-old age group.
- c GAA1 repeat length data was missing for one participant in the 11- to 17-year-old age group.
- d GAA1 repeat length data was missing for three participants in the ≥18-year-old age group.
- e GAA2 repeat length data was missing for five participants in the 5- to 10-year-old age group, 4 of which were because of point mutation in 1 allele.
- f GAA2 repeat length data was missing for seven participants in the ≥18-year-old age group, 4 of which were because of point mutation in 1 allele.
- g Data for SARA were missing for 1 participant.
- ADL = activities of daily living; F = females; FSS = functional staging for ataxia scale; FXN = frataxin gene; mFARS = modified Friedrich's Ataxia Rating Scale; SARA = Scale for the Assessment and Rating of Ataxia.
Procedures and Outcomes
The TRACK-FA protocol was previously published.16 Briefly, the study visit involved clinical, mood and cognitive assessments, blood draws, and magnetic resonance (MR) scan of brain and spinal cord. Participants followed study procedures according to their age group (5–7, 8–10, 11–17, and ≥18 years old).16 The protocol for the 5- to 7-year-old and 8- to 10-year-old age groups differed only according to specific secondary outcome measures not reported here, so these groups are combined as the 5- to 10-year-old group. Self-reported demographic and medical history data were collected from participants. For participants with Friedreich ataxia, genetic information and disease duration were collected. Data collection was standardized, with sites receiving extensive training and the same written study protocol.
MR data were acquired at 3 Tesla using a standardized imaging protocol, which included T1-, T2-, and diffusion-weighted brain imaging; quantitative susceptibility mapping (QSM) of the brain; and T2- and diffusion-weighted imaging and magnetic resonance spectroscopy (MRS) of spinal cord. In participants age 10 years and younger, only T1- and T2-weighted imaging of brain and T2-weighted imaging of spinal cord were acquired to limit the burden in this group.
Of the 7 sites, 5 used a Siemens 3 T Prisma, 1 used a Siemens 3 used T Skyra, and 1 used a Philips Achieva. MRI acquisition parameters are provided in the published protocol.16 Before enrolment, each site undertook rigorous pilot testing of n = 5 volunteers to ensure adequate protocol implementation, site training, and consistent data acquisition. Pilot images were examined by the University of Minnesota imaging team to verify MRI data quality.
Pseudonymized MR data (Digital Imaging and Communications in Medicine (DICOMs)) were uploaded to the Monash XNAT platform. Brain T1 and T2 images were defaced before upload.20 MR analysis was performed at 4 sites (Monash University, RWTH Aachen University, University of Campinas, University of Minnesota). For any given processing pipeline, data were processed by the same site to ensure consistency. IXICO was contracted to independently analyze selected POMs (see Supplementary Material).
The POMs were MR measures selected based on those showing the largest longitudinal effect size in the few small-scale, single-site longitudinal studies available (most unpublished at the time).10, 13, 21, 22 They included cerebrum morphometry: total volume, total white matter (WM) volume, total gray matter (GM) volume; cerebellum morphometry: total volume, superior cerebellar peduncles (SCP) volume; brain diffusion: fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD) in SCP; brain QSM: dentate nucleus (DN) volume and susceptibility; spinal cord morphometry: cross-sectional area (CSA) at C1–C2; spinal cord diffusion: FA, MD, RD, AD at C3–C5; and spinal cord MRS: ratio tNAA/mIns at C4–C5. In participants age 10 years and younger, only cerebrum morphometry, cerebellum morphometry, and spine morphometry were performed. Processing pipeline details are provided in Supplementary Material, and example images for each POM are shown in Figure S1.
Secondary outcomes included clinical scales (assessed only in participants with Friedreich ataxia), namely FSS (scores ≤3 indicate no worse than mild disability, scores of 4 indicate moderate disability, and scores of 5 indicate severe disability); mFARS; Activities of Daily Living (ADL) scale17; and Scale for the Assessment and Rating of Ataxia (SARA).23 Additional analyses of secondary outcome measures are beyond the scope of this report and will be published separately.16 This study is registered with ClinicalTrials.gov (NCT04349514).
Statistical Analysis
Target sample size was determined a priori to detect changes in POMs over the 24-month study period, with an effect size of Cohen's d = 0.3 and power >0.8, and taking into account expected attrition and subgroup stratification.16 This was based on previous single-site longitudinal studies showing that longitudinal effect sizes for the most sensitive imaging metrics ranged from 0.5 to 1.2.10, 13, 21, 22
To verify adequate matching, mean differences between Friedreich ataxia and controls were assessed via 2-sample t tests for years of education and age, and via Fisher's exact test for sex and handedness. Histograms for outcome measures were inspected for outliers or skewness before analysis. Boxplots were used to compare POM distributions across sites, and analysis of covariance analysis adjusted for covariates (control/Friedreich ataxia, sex, age, and education) was used to assess systematic site effects, which were accounted for in subsequent linear models.
To assess the potential effects of omaveloxolone on POMs, we matched treated and untreated participants with Friedreich ataxia according to age, age of onset, sex, gait, and mFARS score, and then assessed differences between these groups using 2-sample t tests (further details are in the Supplementary Material).
Mean differences in imaging metrics between Friedreich Ataxia and controls were assessed using linear regression models adjusted for age, sex, years of education and (for metrics that showed differences between sites) for study site. Relationships between POMs and quantitative clinical factors were assessed via linear regression models, adjusting for the same covariates. The relationship between POMs and FSS was assessed via an ordinal logistic regression model, treating the FSS as ordered categories, adjusting for the same covariates. Pearson correlations between POMs and other clinical factors (GAA1, disease duration) were computed for descriptive purposes. Stratified subgroup analyses were performed for 3 age categories (ages 5–10, 11–17, and ≥18 years old) for all primary analyses. Non-linear trends with respect to age were visualized via local polynomial smoothing using the LOESS method.24 The significance of a non-linear interaction between age and Friedreich ataxia/control status (i.e., whether differences between Friedreich ataxia and control vary with age) was assessed via a permutation test described in the Supplementary Material for each POM. For each analysis, multiple comparisons across POMs were adjusted for false discovery rates (FDR).25 The Benjamini-Yekutieli procedure was used to control overall FDR across dependent tests, such as the association of POMs with multiple clinical outcomes.26 An FDR Q-value <0·05 was considered significant.
Each analysis was performed on fully observed data, with participants removed from a given analysis if they had missing data for a variable needed for that analysis. Missing data for each POM, stratified by age and group, is summarized in Table S1. Analysis was performed using the R environment for statistical computing version 4.1.1. The analysis workflow is available as a reproducible Rmarkdown script. Additional details are in the Supplementary Material.
Results
A total of 285 participants were enrolled and completed some or all assessments. Participants tested at Campinas (n = 12) were excluded because of unforeseen upgrade scanner issues. Four participants were excluded for failing to meet inclusion criteria (all with Friedreich ataxia; 1 for scoring >65 on mFARS; 2 for age of onset >25 years; 1 for disease duration >25 years). Five participants did not complete MRI: 1 because of MRI contraindication and 4 because of MRI-related anxiety. Analyses reported are based on 264 participants (Friedreich ataxia n = 169; control n = 95) (Table 1).
Friedreich ataxia and controls had similar demographic distributions, with no significant differences (p > 0·05) for mean age or years of education, or proportion for sex and handedness. Table 1 summarizes clinical and demographic data for both groups, stratified by age group. Medications are summarized in Table S2.
After all processing was completed, significant site differences (p < 0.05) among the POMs were observed only for brain diffusivity measures (SCP AD, SCP MD, and SCP RD) and for spine AD. Depending on the metric, diffusivities in SCP were 15 to 20% higher in Melbourne than the average value for a given metric across all other sites. This was expected because brain diffusion parameters for the Skyra (in Melbourne) differed from the Prisma because of lower gradient performance.16 No significant differences were found (p > 0.05) among the other sites for brain diffusion measures. Spine AD was ~6% higher in Aachen than at other sites. No specific reason was identified. No significant differences were detected (p > 0.05) among the other sites for spine AD. Based on these findings, the significant site-effects were adjusted for as covariates in the subsequent analyses.
Participants taking omaveloxolone (n = 23) did not differ significantly from those not taking omaveloxolone (p > 0.05) for any POM, after matching for clinical and demographic factors, and therefore, were not considered separately in subsequent analyses.
There was good agreement between POM results obtained with TRACK-FA pipelines and with IXICO pipelines (Fig S2).
Most POMs showed highly significant differences between groups across the entire cohort (p < 0.001) (Table 2). Only AD in SCP, AD in cervical spinal cord, total cerebrum volume, and total cerebrum WM volume were not significantly different; total cerebrum GM volume barely reached significance (FDR = 0.049). The largest differences were observed for brain diffusion in SCP (FA, RD), brain QSM in DN (volume and susceptibility), and cervical spinal cord morphometry (CSA), diffusion (RD), and MRS (tNAA/mIns), with percent differences (either positive or negative) ranging from 26.5 to 53.7%. When stratifying by age, differences in total cerebrum volume and total cerebrum WM volume became significant in adults, but not in the 11- to 17-year-old or 5- to 10-year-old groups (Table S3). Differences were generally largest in adults and smallest in the 5- to 10-year-old group. For example, compared to controls, in the Friedreich ataxia group spinal cord CSA was 37.4% smaller in adults, but only 27.6% smaller in the 11- to 17-year-old group, and 21.4% smaller in the 5- to 10-year-old group.
| Modality | Primary outcome measure | Friedreich ataxia mean (SD) (n = 169) | Ctrl mean (SD) (n = 95) | Adjusted difference | Adjusted difference (%) | Standardized effect | FDR |
|---|---|---|---|---|---|---|---|
| Cerebellum morphometry | Total cerebellar volume (cm3) | 142 (14) | 149 (15) | −7.23 | −4.8 | −0.490 | <0.001 |
| Total SCP volume (mm3) | 877 (101) | 1,023 (124) | −149 | −14.6 | −1.143 | <0.001 | |
| Cerebrum morphometry | Total volume (cm3) | 1,022 (107) | 1,041 (120) | −22.7 | −2.2 | −0.202 | 0.074 |
| Total GM volume (cm3) | 580 (69) | 593 (68) | −13.7 | −2.3 | −0.199 | 0.049 | |
| Total WM volume (cm3) | 442 (55) | 448 (68) | −8.99 | −2.0 | −0.149 | 0.167 | |
| Brain diffusion | Avg SCP FA | 0.469 (0.054) | 0.638 (0.043) | −0.169 | −26.5 | −1.785 | <0.001 |
| Avg SCP MD (10−3 mm2/s) | 0.969 (0.106) | 0.803 (0.073) | 0.172 | 21.4 | 1.381 | <0.001 | |
| Avg SCP RD (10−3 mm2/s) | 0.706 (0.102) | 0.463 (0.058) | 0.249 | 53.7 | 1.696 | <0.001 | |
| Avg SCP AD (10−3 mm2/s) | 1.50 (0.15) | 1.48 (0.14) | 0.019 | 1.3 | 0.131 | 0.367 | |
| Brain QSM | Avg DN susceptibility (ppb) | 95.8 (28.2) | 73.7 (22.3) | 24.8 | 33.7 | 0.878 | <0.001 |
| Total DN volume (mm3) | 1,384 (327) | 1,902 (325) | −516 | −27.1 | −1.257 | <0.001 | |
| Spinal cord morphometry | C1C2 cross-sectional area (mm2) | 45.6 (5.9) | 67.2 (9.7) | −21.8 | −32.4 | −1.703 | <0.001 |
| Spinal cord diffusion | C3C5 FA | 0.511 (0.046) | 0.645 (0.038) | −0.133 | −20.6 | −1.714 | <0.001 |
| C3C5 MD (10−3 mm2/s) | 1.08 (0.09) | 0.947 (0.074) | 0.133 | 14.0 | 1.225 | <0.001 | |
| C3C5 RD (10−3 mm2/s) | 0.756 (0.093) | 0.542 (0.067) | 0.214 | 39.5 | 1.610 | <0.001 | |
| C3C5 AD (10−3 mm2/s) | 1.73 (0.13) | 1.76 (0.13) | −0.029 | −1.6 | −0.223 | 0.076 | |
| Spinal cord MRS | tNAA/mIns ratio | 0.438 (0.109) | 0.803 (0.157) | −0.365 | −45.4 | −1.667 | <0.001 |
- Note: Values that are significant after FDR adjustment (FDR <0.05) are shown in bold. The “adjusted difference” gives the Friedreich ataxia coefficient in the adjusted linear model. The “adjusted difference (%)” gives the Friedreich ataxia coefficient as a percentage of the mean level of the POM for controls. The “standardized effect” gives the ratio of the adjusted difference over the SD of the POM.
- AD = axial diffusivity; DN = dentate nucleus; FA = fractional anisotropy; FDR = false discovery rate; GM, gray matter; MD = mean diffusivity; MRS = magnetic resonance spectroscopy; POMs = primary outcome measures; RD = radial diffusivity; SCP = superior cerebellar peduncles; SD = standard deviation; WM = white matter.
Many POMs showed significant associations with mFARS, SARA, FSS, and ADL scale scores (Table 3). In adults, the strongest and most significant associations were found in SCP (volume, FA, MD, and RD) and in cervical spinal cord (CSA, FA) with direction and strength of association generally consistent across the 4 scales. In the 11- to 17-year-old group, similar associations were observed except for FA in SCP. Correlation coefficients for SCP (volume, RD, and MD) were higher in the 11- to 17-year-old group than in the adult group, reaching 0.64 for the association between RD in SCP and both mFARS and SARA. There were comparatively fewer associations in the 5- to 10-year-old group, with nothing significant at the FDR <0.05 level. However, the ADL scale had a nominally significant (p-value <0.05) association with total cerebellar volume and cervical spinal cord CSA (Table S4).
| Modality | POM names | 11–17 yr (n = 53) | ≥18 yr (n = 96) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mFARS | SARA | ADL | FSS | mFARS | SARA | ADL | FSS | ||
| Cerebellum morphometry | Total cerebellar volume (cm3) | −0.272 | −0.275 | −0.372 *a | −0.375 * | −0.132 | −0.229 * | −0.224 * | −0.186 |
| Total SCP volume (mm3) | −0.451 **a | −0.496 **a | −0.481 **a | −0.492 **a | −0.314 **a | −0.302 **a | −0.324 ** | −0.352 ***a | |
| Cerebrum morphometry | Total volume (cm3) | −0.044 | −0.061 | −0.100 | −0.105 | −0.195 | −0.229 * | −0.239 * | −0.297 ** |
| Total GM volume (cm3) | −0.064 | −0.069 | −0.068 | −0.128 | −0.136 | −0.194 | −0.243 | −0.292 * | |
| Total WM volume (cm3) | −0.015 | −0.045 | −0.127 | −0.066 | −0.231 * | −0.238 ** | −0.205 ** | −0.266 ** | |
| Brain diffusion | Avg SCP FA | −0.304 | −0.186 | −0.201 | −0.279 | −0.427 ***a | −0.343***a | −0.286 ** | −0.303 **a |
| Avg SCP MD (10−3 mm2/s) | 0.571 ***a | 0.636 ***a | 0.416 **a | 0.377 * | 0.220 * | 0.272 **a | 0.077 | 0.159 * | |
| Avg SCP RD (10−3 mm2/s) | 0.637 ***a | 0.638 ***a | 0.460 **a | 0.458 **a | 0.341 ***a | 0.352 ***a | 0.178 * | 0.250 **a | |
| Avg SCP AD (10−3 mm2/s) | 0.357 * | 0.479 ***a | 0.264 | 0.194 | −0.006 | 0.094 | −0.087 | −0.009 | |
| Brain QSM | Avg DN susceptibility (ppb) | 0.148 | 0.197 | 0.277 | 0.278 | 0.077 | 0.069 | 0.331 * | 0.256 |
| Total DN volume (mm3) | −0.185 | −0.299 | −0.124 | −0.207 | −0.116 | −0.110 | −0.093 | −0.074 | |
| Spinal cord morphometry | C1C2 cross-sectional area (mm2) | −0.398 **a | −0.457 **a | −0.422 **a | −0.398 * | −0.365 ***a | −0.348 ***a | −0.230 * | −0.389 ***a |
| Spinal cord diffusion | C3C5 FA | −0.195 | −0.265 * | −0.207 | −0.317 ** | −0.290 ** | −0.358 **a | −0.229 * | −0.290 ** |
| C3C5 MD (10−3 mm2/s) | −0.061 | 0.043 | −0.005 | −0.030 | 0.165 | 0.150 | 0.005 | 0.087 | |
| C3C5 RD (10−3 mm2/s) | 0.060 | 0.171 | 0.125 | 0.155 | 0.223 * | 0.233 * | 0.070 | 0.166 * | |
| C3C5 AD (10−3 mm2/s) | −0.194 | −0.144 | −0.175 | −0.261 | 0.050 | −0.002 | −0.094 | −0.047 | |
| Spinal cord MRS | tNAA/mIns ratio | −0.161 | −0.126 | −0.125 | −0.134 | −0.230 | −0.122 | −0.287 * | −0.153 |
- Note: Correlations are shown, and the significance of the association is assessed using a covariate-adjusted linear model; color shading is determined by the significance and direction of the association, with positive correlations red and negative correlations blue. We use darker colors to denote stronger correlations. Bold values represent statistical significance using raw (unadjusted) p-values: *p < 0.05, **p < 0.01, ***p < 0.001.
- a Significance under the Benjamini-Yekutieli FDR (FDR <0.05) across all POMs and clinical outcomes.
- AD = axial diffusivity; ADL = Activities of Daily Living; DN = dentate nucleus; FA = fractional anisotropy; FDR = false discovery rate; FSS = Functional Staging for Ataxia scale; GM = gray matter; MD = mean diffusivity; mFARS = Modified Friedrich's Ataxia Rating Scale; MRS = magnetic resonance spectroscopy; POMs = primary outcome measures; QSM = quantitative susceptibility mapping; RD = radial diffusivity; SARA = Scale for the Assessment and Rating of Ataxia; SCP = superior cerebellar peduncles; SD = standard deviation; WM = white matter.
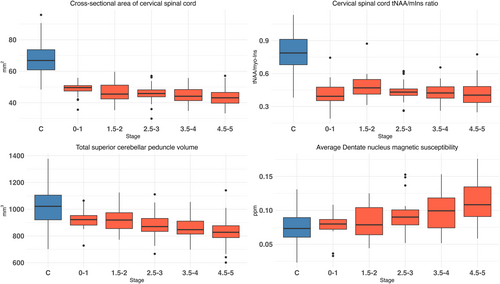
When stratified by disease stage (FSS), some of the POMs were associated with disease stage, whether or not there was a large difference between groups (Fig 1). In the 4 examples shown in Figure 1, cervical spinal cord CSA and tNAA/mIns showed a large difference between Friedreich ataxia and controls. In contrast SCP volume and DN susceptibility showed little difference between the 2 groups. Only cervical spinal cord CSA, SCP volume, and DN susceptibility were significantly associated with FSS. tNAA/mIns showed no association. Similar graphs are provided for all other POMs in Figure S3.
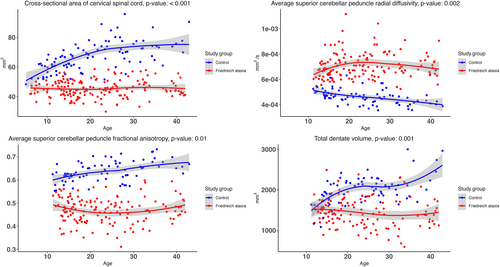
For each POM, we investigated if there was a non-linear interaction effect between Friedreich ataxia/control status and age, that is, whether the trajectory over age differed between Friedreich ataxia and controls after adjusting for mean differences. Four POMs showed a significant interaction with age (FDR <0.05), which were spinal cord CSA; SCP RD and FA; and DN volume (Fig 2). For these 4 POMs, Friedreich ataxia trajectories over age that differed significantly from trajectories in controls, with curves growing farther apart during childhood. DN volume seemed to further increase in controls aged 35 to 45 years, although control data points in that age range were relatively sparse and with greater scatter. When data points in the 35- to 45-year-old age range were removed, the trajectories of DN volume between groups were still significantly different (p = 0.008). Graphs for all the other POMs are presented in Figure S4.
Within Friedreich ataxia, we examined associations between each POM and GAA1 repeat size and disease duration (Table 4). Although no POMs were significantly associated with GAA1 repeat size in the 11- to 17-year-old group, total SCP volume and total cerebrum WM volume were highly significantly associated with GAA1 repeat size in adults (p < 0.001, FDR <0.05). Total cerebrum volume (p < 0.001), total GM volume (p < 0.01), and spinal cord CSA (p < 0.001) also had nominally significant association with GAA1, but did not reach the FDR <0.05 level. Spinal cord MRS had a nominally significant association (p < 0.01) with disease duration, but not GAA1 repeat size. No significant associations were present in children in the 5- to 10-year-old group (Table S5).
| Modality | POM | 11–17 years (n = 53) | ≥18 years (n = 96) | ||
|---|---|---|---|---|---|
| GAA1 | Disease duration | GAA1 | Disease duration | ||
| Cerebellum morphometry | Total cerebellar volume (cm3) | 0.044 | −0.143 | −0.162* | −0.173 |
| Total SCP volume (mm3) | 0.025 | −0.226** | −0.395***a | −0.199** | |
| Cerebrum morphometry | Total volume (cm3) | 0.072 | 0.037 | −0.383*** | −0.144 |
| Total GM volume (cm3) | 0.098 | −0.125 | −0.240** | −0.222 | |
| Total WM volume (cm3) | 0.024 | 0.225 | −0.490***a | −0.044* | |
| Brain diffusion | Avg SCP FA | −0.237 | −0.130 | −0.240 | −0.075* |
| Avg SCP MD (10−3 mm2/s) | 0.058 | 0.334 | 0.087 | −0.091 | |
| Avg SCP RD (10−3 mm2/s) | 0.146 | 0.343 | 0.158 | −0.041 | |
| Avg SCP AD (10−3 mm2/s) | −0.057 | 0.243 | −0.036 | −0.142 | |
| Brain QSM | Avg DN susceptibility (ppb) | −0.108 | −0.036 | −0.006 | 0.327 |
| Total DN volume (mm3) | −0.133 | 0.064 | 0.066 | −0.085 | |
| Spinal cord morphometry | C1C2 cross-sectional area (mm2) | −0.102 | −0.175 | −0.376*** | −0.200** |
| Spinal cord diffusion | C3C5 FA | −0.179 | −0.144 | −0.196* | −0.179* |
| C3C5 MD (10−3 mm2/s) | −0.122 | 0.273** | 0.250 | −0.028* | |
| C3C5 RD (10−3 mm2/s) | 0.009 | 0.281** | 0.244 | 0.039* | |
| C3C5 AD (10−3 mm2/s) | −0.242 | 0.145 | 0.221 | −0.125 | |
| Spinal cord MRS | tNAA/mIns ratio | −0.015 | 0.010 | −0.025 | −0.283** |
- Note: Shading is determined by significance and direction of the association, with positive correlations red and negative correlations blue. Darker colors denote stronger correlations. Bold values represent statistical significance using raw (unadjusted) p-values: *p < 0.05, **p < 0.01, ***p < 0.001.
- a Significance under the Benjamini-Yekutieli FDR (FDR <0.05) across all POMs and associations (GAA1, disease duration).
- AD = axial diffusivity; DN = dentate nucleus; FA = fractional anisotropy; FDR = false discovery rate; GM, gray matter; MD = mean diffusivity; MRS = magnetic resonance spectroscopy; POMs = primary outcome measures; QSM = quantitative susceptibility mapping; RD = radial diffusivity; SCP = superior cerebellar peduncles; WM = white matter.
Discussion
TRACK-FA is the largest prospective, longitudinal, multi-site, multi-modal neuroimaging investigation of individuals with Friedreich ataxia and controls matched for age, sex, handedness, and education. It is the first neuroimaging study to recruit young children (from 5 years old) in numbers sufficient to assess developmental differences in brain and spinal cord. We present cross-sectional analyses of the POMs and examine their associations with clinical outcomes in individuals with Friedreich ataxia.
Comparisons between groups showed significant differences for most POMs. Consistent with previous smaller studies,10, 11, 13 and retrospective meta-analyses of data aggregated from multiple sites,14, 15 individuals with Friedreich ataxia exhibited reduced spinal cord CSA and decreased SCP volume, including in young children. Microstructural analyses of SCP and cervical spinal cord demonstrated reduced FA and increased RD in individuals with Friedreich ataxia, consistent with previous studies.9, 10 Our analyses of the DN show that individuals with Friedreich ataxia show evidence of atrophy and increased susceptibility, consistent with previous studies.21 Analyses stratified by disease stage suggested that DN atrophy is present from early disease stages and then is relatively constant, but DN susceptibility progressively increases in line with disease severity. As susceptibility is a surrogate for iron accumulation, our results are consistent with findings from histological analyses suggesting that iron accumulation is a downstream consequence of DN atrophy.27 Finally, spinal cord MRS data demonstrated that individuals with Friedreich ataxia showed reduced tNAA/mIns ratio, suggesting neuronal loss and myelin abnormalities, consistent with a recent study.10 In accordance with previous studies, we report that many POMs are associated with clinical scale scores, including the mFARS, SARA, and ADL scale, suggesting that these neuroimaging POMs are objective biomarkers of symptom severity.
The TRACK-FA dataset includes individuals spanning 5 to 42 years of age, providing new insights into early-life abnormalities and the neurodevelopmental and neurodegenerative processes that progress with age and disease severity. Notably, distinct morphometric biomarkers, such as spinal cord CSA and SCP volume, significantly differ between groups as early as childhood and become more pronounced with age. Although controls exhibit age-related increases in spinal cord CSA and SCP volume, consistent with typical growth and maturation, individuals with Friedreich ataxia maintain relatively constant levels across age groups, possibly with slight decreases. This pattern suggests a failure to achieve full maturation (hypoplasia), particularly of the spinal cord, followed by neurodegeneration, which aligns with previous neuroimaging11, 12 and histological28 findings. Conversely, differences in other biomarkers, such as total cerebellar volume and total cerebrum WM volume, become evident only in adulthood and later disease stages. This is consistent with prior studies showing that widespread WM and GM damage emerge later through neurodegeneration.12, 14 Additionally, neurochemical markers of neurodegeneration, such as the tNAA/mIns ratio from MRS, show significant differences between groups across all ages. This finding supports recent research suggesting that neurochemical abnormalities may be present before disease onset.10
We report the most comprehensive investigation to date of associations between neuroimaging outcomes and endogenous factors that contribute to disease severity, the best characterized of which include GAA1 repeat size (or genetic severity) and disease duration.3 The size of the pathogenic GAA1 repeat is negatively related both to frataxin levels, the reduction of which precipitates cellular dysfunction and degeneration,1 and therefore, to disease severity.3 However, beyond a GAA1 triplet repeat length “ceiling” of ~700 repeats, clinical severity features are more homogeneous and linear associations between GAA1 repeat length and measures of disease severity are lost.29 Disease duration also contributes to disease severity.3 In adults, many POMs are associated with genetic severity, but these associations are absent in children, even for POMs that significantly differ between control and Friedreich ataxia groups in children. The absence of these associations may be because in children, GAA1 repeat length tends to be ≥700. Consistent with previous work,29 our results suggest that although genetic severity is associated with neuroimaging outcomes to a point, associations plateau at or beyond ~700 GAA triplet repeats. Our findings also indicate that differences between groups for certain POMs poorly correlate with genetic and temporal factors also in adults. The reason why these POMs appear to be less affected by genetic and temporal factors remains unclear, but may be because of differing thresholds for degeneration and/or hypoplasia secondary to genetic drivers.
The TRACK-FA study boasts several notable strengths that distinguish it from previous studies. First, data collection was prospective, following a standardized protocol across all sites.8 Second, large sample size and inclusion of young children allowed stratification by age, providing new insight into the role of developmental versus degenerative changes. Third, the longitudinal, multi-modal neuroimaging protocol offers a comprehensive assessment of disease-related changes at various levels of the brain and spinal cord over time. Last, independent assessment of neuroimaging outcomes by IXICO corroborated our findings, underscoring the robustness of these measures.
Our results offer significant implications for identifying sensitive biomarkers of disease progression in Friedreich ataxia.4, 5 Assessing changes in populations experiencing more rapid decline, such as young children and those in early disease stages, is crucial for identifying sensitive biomarkers.4 Furthermore, young children represent a subgroup for which sensitive biomarkers are especially needed. Clinical scale reliability is more limited in this group compared to adults, as scores are confounded by development of the cerebellum and motor systems.6
Several limitations should be noted. First, although these analyses reveal associations across various age groups and disease severities, they offer a cross-sectional snapshot. Because TRACK-FA is longitudinal in nature, subsequent follow-up analyses will enable quantification of the rate of change in POMs within and between groups, potentially identifying the most sensitive biomarkers of disease progression. Second, although TRACK-FA is unique in its assessment of neuroimaging outcomes in children, the number of very young participants is relatively small. This may have limited our ability to detect associations between POMs and clinical parameters. Third, generalizability of our findings is limited to the population of individuals who met the inclusion and exclusion criteria. The cohort is skewed to earlier disease stages. Almost 75% were in mild to moderate FSS categories, therefore, indicating that most of the cohort was ambulatory. This contrasts with the broader Friedreich ataxia population, in which individuals become wheelchair-dependent on average 10 to 15 years after symptom onset.30
TRACK-FA aims to unravel the natural progression of the disease in the central nervous system. By assessing participants across ages and disease stages, including young children, we conclude that MR scans of brain and spinal cord can detect significant abnormalities even in the earliest disease stages, driven by abnormal development, especially in the spinal cord. Moreover, our results suggest that structures higher up in the spino-cerebello-thalamo-cortical pathway, such as the cerebrum, tend to change later in the disease course. Ultimately, based on this work, in conjunction with forthcoming 12- and 24-month longitudinal follow-up, TRACK-FA will contribute to the identification of the most sensitive neuroimaging biomarkers of progression, according to age and disease stage. This will lay the critical groundwork required for advancing these biomarkers into future clinical trials.
Acknowledgements
TRACK-FA is funded by grants from the Friedreich's Ataxia Research Alliance (FARA, USA) to each of the academic sites and IXICO with financial support from Takeda Pharmaceuticals Company, Novartis Gene Therapies, IXICO, Larimar Therapeutics Inc., and PTC Therapeutics. FARA does not use grant numbers. We gratefully thank all of the individuals for their participation in the study and FARA, USA for their help with Friedreich ataxia participant recruitment. We thank and acknowledge FARA, Takeda Pharmaceuticals Company, Novartis Gene Therapies, IXICO and PTC Therapeutics for support in funding and for their participation in the TRACK-FA Neuroimaging Consortium, including providing advice and feedback on study design, implementation, and analysis of outcomes. We thank the following industry partners for their valuable contributions to the TRACK-FA Neuroimaging Consortium. From FARA: Dr M. Rai. From PTC Therapeutics: Drs J. Cherry, A. Wang, and M. Weetall. From Novartis: Drs A. Misko and A. Sverdlov. From Takeda: Drs C. Salinas, O. Yardibi, and C. Yeh. Study data were collected and managed using REDCap electronic data capture tools hosted and managed by Helix (Monash University, Australia). Raw imaging data (DICOMs) are stored and managed using XNAT, a software framework for managing neuroimaging laboratory data hosted by Monash (MXNAT), and Monash Biomedical Imaging (MBI-XNAT) (Monash University, Australia). We thank the research coordinators across all TRACK-FA sites for their assistance in participant recruitment and testing. We are grateful to D. Hutter (study coordinator at the University of Minnesota, USA); A. Tomaselli (research assistant at Monash University, Australia); G. Tai (Research Assistant at the Murdoch Children's Research Institute); M. Coker (study coordinator at the University of Florida, USA); C. Rampal (study coordinator at McGill University, Canada); C. Birnbaum (clinical research assistant at the Children's Hospital of Philadelphia); S. Ward (clinical research assistant at the Children's Hospital of Philadelphia, USA); V. Kaufmann (study coordinator at the Children's Hospital of Philadelphia, USA); Dr M. Kim, (psychologist at the Children's Hospital of Philadelphia, USA); Dr L. Blaskey, (psychologist at the Children's Hospital of Philadelphia, USA); A. Grgic (study coordinator at RWTH Aachen University Hospital); S. Schawohl (study coordinator and PhD student at RWTH Aachen University Hospital); Dr R. Dadsena (post-doc researcher at RWTH Aachen University Hospital); and Mr. S. Mirzazade (MRI technologist at RWTH Aachen University Hospital). Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
Author Contributions
N.G.K., L.A.C., M.B.D., I.D., J.F., M.F., I.H.H., K.R., C.L., and P.G.H. contributed to the conception and design of the study; L.A.C., P.G.H., E.F.L., H.B., I.A., M.C., D.K.D., M.B.D., I.D., M.F., A.S.G., W.G., I.H.H., J.J., J.L., M.A.L., D.R.L., T.H.M., A.R.M.M., M.Pan., M.Pap., R.G.P., K.R., T.J.R.R., T.P.R., S.R., D.A.R., S.S., J.B.S., S.H.S., and V.G.S. contributed to the acquisition and analysis of data; N.G.K., L.A.C., P.G.H., E.F.L., C.L., H.B., and J.L. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
J.F. is an employee of the FARA, a funder of the study reported here; FARA has received funding from Takeda, PTC Therapeutics, Novartis Gene Therapies, IXICO Technologies and Larimar Therapeutics for the TRACK-FA study reported in this publication. M.Pap., M.A.L., R.G.P., V.G.S., and A.S.G. are full-time employees of IXICO, a contract research organization for the study reported here. All other authors declare no conflicts of interests.
Open Research
Data availability
The TRACK-FA Neuroimaging Consortium is committed to data sharing with the broader research community within 2 years of study completion.




