Increased blood reactive oxygen species and hepcidin in obstructive sleep apnea precludes expected erythrocytosis
Jihyun Song and Krishna M. Sundar co-first authors and contributed equally to this work.
[Correction added on 10 February, 2025, after first online publication: The copyright line was changed.]
Abstract
Obstructive sleep apnea (OSA) causes intermittent hypoxia during sleep. Hypoxia predictably initiates an increase in the blood hemoglobin concentration (Hb); yet in our analysis of 527 patients with OSA, >98% did not have an elevated Hb. To understand why patients with OSA do not develop secondary erythrocytosis due to intermittent hypoxia, we first hypothesized that erythrocytosis occurs in these patients, but is masked by a concomitant increase in plasma volume. However, we excluded that explanation by finding that the red cell mass was normal (measured by radionuclide labeling of erythrocytes and carbon monoxide inhalation). We next studied 45 patients with OSA before and after applying continuous positive airway pressure (CPAP). We found accelerated erythropoiesis in these patients (increased erythropoietin and reticulocytosis), but it was offset by neocytolysis (lysis of erythrocytes newly generated in hypoxia upon return to normoxia). Parameters of neocytolysis included increased reactive oxygen species from expanded reticulocytes' mitochondria. The antioxidant catalase was also downregulated in these cells from hypoxia-stimulated microRNA-21. In addition, inflammation-induced hepcidin limited iron availability for erythropoiesis. After CPAP, some of these intermediaries diminished but Hb did not change. We conclude that in OSA, the absence of significant increase in red cell mass is integral to the pathogenesis, and results from hemolysis via neocytolysis combined with inflammation-mediated suppression of erythropoiesis.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is highly-prevalent sleep disorder1 characterized by recurrent obstruction of airflow due to upper airway collapse during sleep,2 leading to chronic intermittent hypoxia (CIH).3 OSA is treated with continuous positive airway pressure (CPAP).4 OSA-related CIH contributes to hypertension, obesity, diabetes, thromboses, and cancer-related deaths.5
CIH-driven activation of oxygen-sensing carotid glomus cells by hypoxia inducible factors (HIFs) leads to sympathetic activation and adrenal medullary secretion of catecholamines, leading to hypertension.6 However, the mechanisms behind other pathophysiologic consequences of OSA are largely unknown.
We describe the effects of OSA on the erythron and on peripheral blood cells. Erythron is controlled by hypoxia to assure optimal tissue oxygen delivery.7 HIFs (HIF-1 and HIF-2) are dimers of α and the shared β subunit and regulate the transcription of hypoxia-controlled genes.8 The ubiquitously expressed HIF-1 upregulates more than 2% of genes in endothelium,9 while HIF-2 is more tissue-restricted and is the principal regulator of erythropoietin (EPO).10 The EPO response explains erythrocytosis in chronic lung disease, Eisenmenger syndrome, pulmonary arterial venous shunts, high oxygen affinity hemoglobin and methemoglobin, or low ambient atmospheric oxygen.11 Indeed, we found that HIFs-regulated genes are changed in OSA as expected.12 The HIFs' effect on erythropoiesis is conventionally measured by changes of peripheral blood hemoglobin concentration (Hb) and hematocrit (Hct); however, Hb and Hct depend not only on red cell volume (RCV) but also on plasma volume (PV).11 Concomitant measurements of RCV and PV may show unexpected results as reported in Tibetan Sherpas who have normal Hb and Hct at high altitude but increased RCV and PV; thus, their true erythrocytosis is masked by elevated PV.13
Although OSA is generally considered a cause of erythrocytosis,14 we found a very low prevalence of high Hb in patients with OSA. In 2016, we reported that 1.7% of the 527 patients with OSA had high Hb.15 Subsequent meta-analyses of Hb and Hct in patients with OSA showed that the prevalence of high Hb was variable but low.16 Further, these meta-analyses were limited by inclusion of studies that did not factor in the effect of any OSA treatment to validate that high Hb was indeed OSA-related, variability in criteria used to categorize high Hb, and exclusion of confounders such as smoking.
To understand the mechanisms of CIH involved in the paucity of erythrocytosis in OSA, we built on a murine neocytolysis model wherein the reversal from sustained hypoxia to normoxia is somewhat analogous to what is seen in OSA. Blood effects in this model were attributable to neocytolysis, that is, hemolysis that preferentially affects young red blood cells (RBCs) (neocytes). This process of neocytolysis results in transient overcorrection of high Hb and RCV that occurred during hypoxia17 associated with expanded mitochondria and their reactive oxygen species (ROS) in reticulocytes from decreased HIF-mediated mitophagy.18 RBCs generated during sustained hypoxia had low antioxidant catalase due to downregulation of CAT (encoding catalase) by hypoxia-induced microRNA-21 (miR-21).18 Based on the similarity to transitions of hypoxia to normoxia seen in OSA, we hypothesized that neocytolysis might also explain the absence of erythrocytosis in OSA.
Patients with OSA have ROS-mediated cell injury and elevated plasma inflammatory markers, including C-reactive protein, IL-6, TNF-α, IL-8, and NF-κB.19 The role of these in injury and inflammation in multiple organs has been described,20 and increased levels of inflammatory cytokines including IL-6, TNF-α, and the master inflammatory transcription factor NF-κB are known to suppress erythropoiesis. Inflammation also induces hepcidin (the principal regulator of iron availability) which suppresses erythropoiesis by blocking iron release from macrophages.21 We hypothesized that the absence of high Hb in OSA might also be caused by inflammation-mediated suppression of erythropoiesis.22 We prospectively evaluated these processes before and after CPAP.
2 METHODS
We conducted a retrospective analysis of 552 patients evaluated for OSA at the University of Utah Sleep-Wake Center, Salt Lake City from January 2013 to October 2015. Hematologic, other laboratory and clinical parameters were available for 527 patients prior to and after CPAP therapy. There were 273 males (51.8%). Patients with suspected OSA were evaluated by a sleep medicine provider followed by formal testing for OSA with a polysomnography (PSG) or home sleep test (HST). OSA severity was defined based on apnea-hypopnea index (AHI) as mild (5–15/h), moderate (15–30/h), or severe (>30/h). From the PSG or HST, information on AHI, lowest oxygen saturation (SpO2), time spent below SpO2 <89% and 4% oxygen desaturation index (ODI) was obtained. The effect of altitude on Hb at 1000 m showed the average Hb is 0.62 g/dL per 1000 m of altitude.23 Salt Lake City is located at an altitude of 1450 meters, resulting in higher Hb values compared to sea level (approximate a 1 g/dL increase for every 1500 m), with the sex difference in Hb persisting at altitude;24 high Hb was defined using 2008 WHO criteria available at the time of this study as Hb > 18.5 and 16.5 g/dL in males and females, respectively. Blood samples were collected before and after 3 months CPAP. Optimal CPAP therapy was defined as usage of CPAP for more than 4 h/night for 70% of the nights and a machine AHI ≤5 at after 3 months of CPAP.
2.1 Measurement of hemolysis using ETCOc
We assessed hemolysis by measuring carbon monoxide (CO) concentration in end-tidal exhaled air (ETCOc) using CoSense ETCO monitor (Capnia). Most of the CO in exhaled air is derived from catabolism of Hb and increased ETCOc indicates hemolysis.25
2.2 Assessment of red cell mass and plasma volume
- CO rebreathing technique: Each subject was studied in a preprandial state in the daytime. Eight patients with OSA (two mild, two moderate, and four severe) had measurement of RCV determined using a CO rebreathing device (Detalo Performance).26 Individuals rested for 20 min in the supine position before each measurement during when a catheter was inserted in an antecubital vein. Subsequently, individuals breathed 100% oxygen for 4 min to flush nitrogen from the airways. At 3 min of oxygen breathing, 2 mL blood was sampled and analyzed immediately for percentage carboxyhemoglobin as a portion of total hemoglobin (% HbCO). Then, a bolus of 1.2 mL kg-body weight of 99.997% chemically pure CO was administered into the breathing circuit. Individuals re-breathed this gas mixture for 10 min in a closed circuit where oxygen is added on a demand basis. An additional 2 mL blood sample was obtained and analyzed. The change in % HbCO was used to calculate RCV and PV derived from measures of Hbmass and Hct. RCV measurements using this technique were compared to the means of age, sex, height, and weight-matched controls at similar altitude of Salt Lake City.
- Radioactive red-cell labeling: We determined the RCV, PV, and total blood volume (TBV) by labeling erythrocytes with 111 indium oxine (111In-oxine) and measuring the radio-activity of the blood sample after the mixing of administered labeled sample in the circulation using dilution.27, 28 TBV was established by RCV/Hct and PV was determined by subtracting RCV from TBV.27, 28
2.3 Measurement of hematological parameters
EPO in frozen plasma was measured by ARUP lab at University of Utah using quantitiative chemiliuminescent immunoassay. Hct was determined by micro-hematocrit centrifuge methods using micro-capillary tubes. Reticulocytes were enumerated by thiazole orange (BD Bioscience) and analyzed by FACS.18
2.4 Blood separation
Granulocytes, platelets, and reticulocytes were separated by density gradient method using Histopaque (Sigma).29 RNA was isolated using Tri-reagent (Molecular Research Center).
2.5 FACS analysis
To measure ROS in reticulocytes and RBCs, Cy5-CD71, PE-CD235a, and 5 μM of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (Invitrogen) was used. For mitochondrial mass, 25 nM of MitoTracker® Deep Red FM (Invitrogen) with FITC-CD71 and PE-CD235a were used. ROS in other blood cells was measured after lysing RBCs.18 PE-conjugated surface markers against CD3-T-cell, CD19-B-cells, CD14-monocytes, and CD45-granulocytes were added and cells enumerated.18
2.6 Transcript analysis
Total RNA was isolated and reverse-transcribed by SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen). Transcripts in granulocytes, reticulocytes, and platelets were measured by real-time PCR with Taqman probes (Thermofisher) and expression levels were calculated as described.18 Taqman probe: EGLN1: Hs00254392_m1, CAT: Hs00156308_m1, HK1: Hs00175976_m1, PDK1: Hs01561850_m1, SOD2: Hs00167309_m1, SLC2A1: Hs00892681_m1, TFRC: Hs00951083_m1, NOX2(CYBB): Hs00166163_m1, BNIP3L: Hs00188949_m1; mRNAs in reticulocytes and platelets were normalized against GAPDH and in granulocytes as against HPRT1. miR-21 and U6snRNA were measured by TaqMan® miR-21 reverse transcription kit (Applied-Biosystems) using Taqman primers/probes miR-21 (Assay ID: 000397) and U6snRNA (Assay ID: U6 snRNA).
2.7 Catalase enzyme activity
Catalase enzyme activity were measured by Catalase Colorimetric Activity Kit (Invitrogen) as described.18
2.8 Hepcidin and ferritin measurements
Frozen ACD plasma samples were used to determine hepcidin and ferritin by DRG Hepcidin 25 HS and Ferritin (Abcam) ELISA kits, respectively.
2.9 Iron metabolism parameters
ACD plasma samples were used to determine soluble transferrin receptor (sTfR), unsaturated iron- binding capacity (UIBC), total iron binding capacity (TIBC), and transferrin saturation (TSAT). Plasma iron (Fe), soluble transferrin receptor (sTfR), and UIBC were measured with Cobas 8000 analyzer (Roche/Hitachi); TIBC was calculated using UIBC and plasma iron, TSAT was calculated by plasma iron divided by the TIBC and expressed as percentages.
2.10 Erythroferrone
Plasma human erythroferrone concentrations were measured by an enzyme-linked sandwich immunoassay.30
2.11 Statistical analyses
Paired or unpaired t-tests were used to determine the statistical significance of the results. Correlation analyses were performed by the GraphPad prism (GraphPad Software Inc.).
3 RESULTS
3.1 A retrospective analysis of patients with OSA to assess Hb and Hct
Our analysis first included retrospective analysis of 552 OSA nonsmokers who had Hb values available within a year of their OSA diagnosis.15 In follow up of this observation, we analyzed those 347 patients whose OSA was controlled by CPAP. We compared their baseline Hb values before treatment of OSA to Hb values after CPAP therapy. These included 174 males (mean age ± SD: 58 ± 13.1 years) and 173 females (58.6 ± 15.6 years). Of these, 16/347 had baseline Hb values that were elevated to the erythrocytosis range (Table S1). Eleven of the sixteen were being treated with testosterone, a known cause of erythrocytosis31 or supplemental oxygen during the day for underlying chronic pulmonary or cardiac disease; these were removed from our analysis.15 Only 5/347 patients had high Hb without any coexisting testosterone or oxygen use. Of these five, two had persistent high Hb despite correction of OSA for >1 year and were referred for further evaluation of other causes of erythrocytosis. This left 0.86% (3/347) patients whose Hb normalized with CPAP, as an estimate of prevalence of OSA-related high Hb, not attributable to other causes.
3.2 Relationship of Hb with polysomnographic parameters
Characteristics of analyzed OSA patients based on different sleep apnea categories are shown in Table S2. Mean Hb values were similar in all patients irrespective of the severity of OSA. Correlation of Hb with oximetric indices was analyzed for the entire cohort of 347 and for differing categories of OSA severity. No correlation of Hb with AHI, (r = .047; p = .37), average SpO2 (r = .04; p = .43), 4% ODI (r = .26; p = .64), time spent below 89% SpO2 (r = .065; p = .25), or lowest SpO2 (r = .099; p = .06) was found, and neither were there any correlations between any of the oximetric indices and Hct.
3.3 Study subjects
To study the molecular mechanisms of the absence of erythrocytosis in OSA and effect of CPAP treatment, 45 patients with OSA (30 males and 15 females; 19 mild, 13 moderate, and 13 severe) were studied before and after CPAP. Mean age was 51.6 ± 12.7 years with average AHI of 25.4 ± 22. PSG was analyzed for oximetric abnormalities (lowest SpO2, time spent below 89% SpO2, and ODI of 3% and 4%).
3.4 Assessment of erythropoietic activity
In these 45 patients, plasma EPO was measured before initiation and after 3 months of nightly CPAP. Baseline EPO concentrations in OSA patients were higher than in controls, and increased EPO diminished after CPAP (Figure 1A,B), in strong support of sleep-related hypoxia as a cause of increased EPO. Moreover, EPO correlated with time spent below 89% SpO2 (Figure 1C). The reticulocyte numbers were higher in OSA compared to controls and normalized with CPAP (Figure 1D,E). A weak correlation without statistical significance was observed between EPO and reticulocyte counts, suggesting that factors other than EPO contribute to the heterogeneity of reticulocyte count in OSA patients (Figure S1). However, Hcts were not significantly different between OSA patients and controls (Figure 1F) and did not change after CPAP (Figure 1G). Hcts correlated with time spent below 89% SpO2 and with 3% ODI (Figure 1H,I).
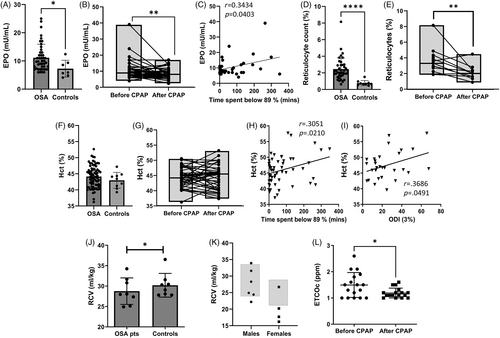
3.5 Absence of erythrocytosis in patients with OSA
We used two methods to measure RCV: (1) Inhaled CO technique26: Eight patients had measurement of RCV using the CO technique and had less RCV compared to age, gender, and comparable residence altitude matched controls (Figure 1J). (2) Radioactive red–cell labeling method28: In the past, standard measurements of RCV and PV used labeling the RBCs with radioactive isotopes.27, 28 Due to the limited availability of chromium-51 (51Cr), 111In-oxine is now being largely used.28 Because of concerns about radiation exposure, this approach is no longer available in the United States. Thus, we collaborated with our colleagues at Olomouc (altitude of 219 m), Czech Republic, where an independent RCV and PV analysis of patients with OSA using 111In-oxine was performed. One of six males was slightly above and another slightly below normal range of RCV; four males were normal. In four female patients, three of four had lower RCV than normal range and one was normal (Figure 1K). TBV and PV determined by CO technique was lower in OSA patients compared to controls (Figure S2A,C). Two of six males and one female were in normal range of TBV, and PV assessed by 111In-oxine-labeling method (Figure S2B,D).
3.6 Assessment of hemolysis using ETCOc measurements
We assessed hemolysis by measuring ETCOc in exhaled air25 in 17 untreated and 17 treated patients (Figure 1L). In normal adult subjects, ETCOc is <1 ppm.25 The instrument does not quantitate the ETCOc <1 ppm but reports exact numeric values if >1 ppm. The mean ± standard error of mean (SEM) values of ETCOc in 17 untreated OSA patients was elevated at 1.49 ± 0.11 ppm and declined to 1.19 ± 0.05 ppm in CPAP treated patients (Figure 1L).
3.7 ROS in reticulocytes and RBCs before and after CPAP
ROS were higher in OSA reticulocytes and RBCs compared to controls (Figure 2A,F), as was the percentage of ROS-positive OSA reticulocytes and RBCs (Figure 2B,G). These parameters decreased after CPAP (Figure 2D,E,I,J). The increase in ROS in reticulocytes and RBCs was similar to that documented in our murine neocytolysis model.18 The levels of ROS and percentage of ROS-positive reticulocytes and RBCs correlated with time spent below 89% SpO2 (Figure 2C,H), suggesting that ROS accumulation correlates with OSA severity.

3.8 Catalase transcript and enzyme activity before and after CPAP
Both CAT transcript in reticulocytes and catalase activity in RBCs increased after CPAP (Figure 2K,M). The miR-21 level, which downregulates CAT mRNA, showed weak nonsignificant inverse correlation with CAT transcript (r = −.3898, p = .0893) and decreased after CPAP (Figure 2O). Weak and moderate inverse correlations were observed between CAT transcript and catalase activity, respectively, and the severity of OSA (Figure 2L,N), suggested that patients with severe OSA accumulate more ROS due to lower catalase activity resulting from upregulated miR-21.
3.9 ROS in other circulating blood cell lineages
We detected increased ROS in monocytes, granulocytes, B-cells, and T-cells in OSA (Figure S3A,C,E,G), confirming that oxidative stress, as a characteristic feature of the disease, is not limited to the erythroid lineage. These elevated ROS levels were normalized with CPAP (Figure S3B,D,F,H).
3.10 Mitochondrial mass in blood cells before and after CPAP
Mitochondria are the major source of reticulocyte ROS.32 Mitochondrial mass in reticulocytes decreased after CPAP (Figure S4A,B). However, mitochondrial superoxide (O•−) measured by Mito–Sox was not changed after CPAP (data not shown) unlike what was demonstrated in the murine neocytolysis model.18
3.11 Dysregulated HIF target genes expression in OSA
Acute hypoxia stabilizes HIF-1α and HIF-2α; however, chronic sustained hypoxia decreases HIF-1α degradation while HIF-2α protein remains stable.33, 34 To evaluate HIF transcriptional activity during OSA-related CIH, expression levels of HIF target gene before and after CPAP were measured in different blood cell lineages (reticulocytes, granulocytes, and platelets). We selected seven genes which are upregulated by HIF (HK1 encoding hexokinase 1, SLC2A1 encoding glucose transporter 1, PDK1 encoding pyruvate dehydrogenase kinase, TFRC encoding transferrin receptor, SOD2 encoding superoxide dismutase 2, NOX2 encoding NADPH oxidase 2, and EGLN1 encoding prolyl hydroxylase 2). In reticulocytes, 5 out of these 7 HIF target gene transcripts increased after 3 months of CPAP suggesting that HIF activity was lower during CIH but normalized after OSA correction by CPAP (Figure S5A). In granulocytes, HK1, PDK1, NOX2, and EGLN1 increased after CPAP while TFRC decreased (Figure S5B). In platelets SLC2A1, NOX2, and EGLN1 transcripts were higher after CPAP while the other genes' transcripts were not changed (Figure S5C).
3.12 OSA and inflammation
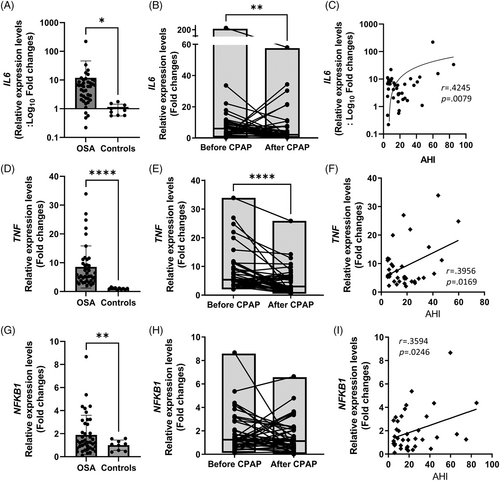
To evaluate possible erythropoietic suppression by OSA-related inflammation, we assayed the transcripts of selected inflammatory markers in granulocytes. The transcripts of inflammatory markers, including IL6, TNF, and NFKB1, were all increased compared to controls, (Figure 3A,D,G), correlated with the AHI (Figure 3C,F,I), and normalized after CPAP except for severe OSA patients (Figure 3B,E,H).
3.13 Hepcidin and erythroferrone before and after CPAP
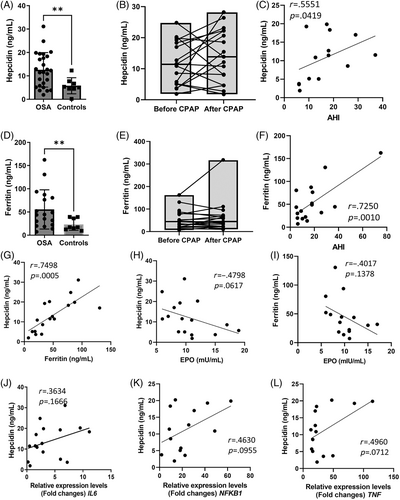
Serum hepcidin was elevated in OSA (Figure 4A) and correlated with AHI (Figure 4C). In most of the patients, hepcidin did not change after CPAP (Figure 4B). However, it markedly increased in some of the most severe OSA patients. (Figure 4B). Serum ferritin was higher in OSA than in controls (Figure 4D) and correlated with several indices of OSA and CIH severity, AHI, time spent below 89% SpO2, lowest SpO2, 4% ODI, and 3% ODI (Figure 4F and Figure S6A–D). However, ferritin did not change after CPAP (Figure 4E). As expected, hepcidin correlated positively with ferritin (Figure 4G); inverse correlation between EPO and hepcidin or ferritin was moderate but did not reach statistical significance (Figure 4H). Weak or moderate nonsignificant positive correlation between hepcidin and inflammatory genes' transcripts in granulocytes was observed (Figure 4J–L). Erythroferrone is produced by erythroid progenitors. It is upregulated by EPO and suppresses hepcidin production. In OSA, erythroferrone was within the normal range30 and positively, but nonsignificantly correlated with EPO (Figure S7A). OSA patients with higher erythroferrone spent greater time below 89% SpO2 (Figure S7B); this parameter also positively correlated with EPO (Figure 1C). Erythroferrone changes after CPAP were variable (Figure S7C), suggesting that sustained inflammation drives stress erythropoiesis in severe OSA.
3.14 Iron metabolism
Considering that hypoxia, increased erythropoiesis, EPO, and inflammation all influence iron homeostasis, we examined whether OSA affects other parameters of iron metabolism. Plasma Fe was lower in OSA compared to controls (Figure S8A) and correlated with Hct (Figure S9A). sTfR was higher in OSA than controls (Figure S8C) consistent with increased and/or iron deficient erythropoiesis. OSA patients had lower TSAT than controls (Figure S8E), as expected with increased inflammation. TSAT was positively correlated with EPO but inversely correlated with sTfR (Figure S9B,C). Similar to hepcidin and inflammatory markers, iron parameters changed differentially after CPAP (Figure S8B,D,F).
4 DISCUSSION
The molecular events that lead to OSA pathophysiology are not fully defined. Despite substantial hypoxia during OSA-associated CIH, high Hb is infrequent.11, 15 After we reported that 1.7% of 527 patients with OSA had high Hb,15 two meta-analyses concluded that the prevalence of high Hb in OSA patients was low (2% or less) but higher in those with severe OSA. However, only 434 patients from 10 studies were included in the follow-up Hb and Hct measurements after 1 night to 12 months of CPAP therapy.16 Although some of these studies reported decreased Hct after CPAP, its effect was small and often in the range of normal Hb values.35 Some of these studies included smokers. In one of the meta-analyses, a significant CPAP effect on Hct was noted; however, it occurred following a night of CPAP use, suggesting the presence of hemoconcentration.35 In our present report, we specifically excluded these confounders and confirmed a low prevalence of high Hb in OSA. None of these studies assessed whether erythrocytosis may be masked by increased PV, that is, masked erythrocytosis.13 Here, we demonstrate for the first time, using two separate methods that true erythrocytosis is absent in the majority of OSA subjects and that OSA is not a common cause of unexplained erythrocytosis.
We document here that, on the average, there was a small increase in erythroid stimulus and a modest enhancement of erythropoiesis in OSA manifested by elevated EPO and reticulocytosis. Both EPO and reticulocyte counts decreased to normal following CPAP compared to before CPAP. Despite somewhat increased erythropoietic drive in OSA, Hb did not increase. To account for this apparent paradox, we demonstrated two independent mechanisms that oppose erythrocytosis in OSA: (1) hemolysis (neocytolysis) and (2) concomitant suppression of erythropoiesis by hepcidin.
Firstly, neocytolysis-mediated hemolysis in OSA explains the absence of erythrocytosis. Because conventional markers of hemolysis such as indirect bilirubin, serum haptoglobin, and LDH are not sensitive enough to detect low levels of hemolysis in OSA,36 we utilized an FDA-approved ETCOc hemolysis assessment25 and found increased values for OSA patients. This, along with reticulocytosis, indicates decreased RBC survival likely mediated by an increase ROS in reticulocytes and RBCs. Other non-erythroid blood cells, that is, monocytes, granulocytes, B-cells, and T-cells, may contribute to elevated ROS in the circulation. This, in turn, may further aggravate destruction of RBCs due to their insufficient antioxidant defense, as discussed below. These aberrations normalized after CPAP and were similar to the changes seen in our hypoxia-to-normoxia murine neocytolysis model.18 A crucial role of HIFs in mediating RBC abnormalities during transitions from hypoxia to normoxia was also demonstrated in this model,18 wherein HIF augmentation ameliorated hemolysis along with diminishing ROS generation, mitochondrial mass, catalase, and miR-21 changes.18 In OSA, the percentage of ROS-positive reticulocytes were less than ROS-positive RBCs. Increased ROS in RBCs can be explained by (1) diffusion of ROS into RBCs from reticulocytes which is observed in murine neocytolysis model, (2) overall younger age of RBCs in OSA circulation relative to controls that may contribute to higher level of ROS-positive RBCs, and (3) higher activity of NADPH oxidases, and relatively lower activity of antioxidant defense, principally catalase, in these younger RBCs. Catalase was the major antioxidant defense in RBC in the murine neocytolysis model.18 CPAP increased catalase, indicating an insufficient catalase activity to scavenge increased ROS in OSA. In murine neocytolysis model, increased reticulocyte ROS occurred from expanded mitochondria.18 We show that in OSA, the reticulocyte mitochondrial mass decreased following CPAP. We also observed increased ROS in other non-erythroid blood cells including T-cells, B-cells, granulocytes, and monocytes. CIH of OSA is characterized by increased ROS, particularly mitochondrial ROS, which has been demonstrated in multiple organs including palate muscles,37 pancreatic β-cells,38 and brain.39 The role of increased ROS in various types of leukocytes is only now being recognized to be associated with thrombotic, atherosclerotic, and inflammatory changes.40
Secondly, the absence of erythrocytosis in OSA is partly due to the suppression of erythropoiesis by increased inflammation and hepcidin. OSA increases biomarkers of systemic inflammation, including C-reactive protein, IL-6, TNF-α, IL-8, with NF-κB activation.41 Since increased inflammation is also interconnected with increases in ROS,42 we measured ROS in other blood cells and observed increased ROS in various types of leukocytes (see above paragraph). Inflammation induces hepcidin, which suppresses erythropoiesis by limiting iron availability for the erythron.43 Consistently with this mechanism, we observed high hepcidin, high ferritin but low Fe and TSAT in OSA compared to controls. The levels of erythroferrone, which is secreted by EPO-stimulated erythroid precursors and suppresses hepcidin transcription in the liver,44 were within the normal range, though substantial interindividual differences among OSA patients were noted. The erythroferrone and plasma EPO at a high and upper normal range we observed in severe OSA would be expected to augment erythropoiesis. However, erythropoiesis in OSA is suppressed by inflammation and restricted iron supply via elevated hepcidin. In this setting, erythroferrone is probably not high enough to suppress hepcidin. Similar findings are observed in the anemia of cancer and inflammation.45 Our data suggest that in OSA, hepcidin stimulation by inflammation supersedes the suppressive effects of increased erythropoiesis. While we expected normalization of hepcidin to accompany the decreased inflammation, neither hepcidin nor other iron status parameters changed after CPAP. We speculate that the anti-inflammatory effects of CPAP were not strong enough to decrease hepcidin. One counteracting mechanism opposite to inflammation augmenting hepcidin levels is hypoxia as the HIFs' activity is an independent mechanism of decreasing hepcidin.46 The relative roles of decreased red cell survival from increased ROS versus erythropoiesis suppression by inflammation and restricted iron supply via elevated hepcidin cannot be surmised from our data but we submit both these processes play important roles in preventing erythrocytosis in OSA.
HIFs are involved in maintaining the redox balance. In rodent CIH models of hypertension, carotid bodies' HIF-1 is increased while HIF-2 is decreased.47 HIF activation is estimated by quantitation of transcripts of HIF-regulated genes. CPAP corrected some of the abnormal gene expression, but others persisted, likely explained by HIF-mediated epigenetic modifications documented in hypoventilation syndrome and in OSA.48 In rodents, CIH induces DNA 5′UTR methylation, causing transcriptional dysregulation48 and in human monocytes, methylation of genes are regulated by peroxisome proliferator-activated receptors.49 This suggests that CIH induces transcription of certain HIF target genes in a tissue-specific manner resulting in a heterogeneous HIF-target gene expression.
In summary, we describe OSA-associated changes in blood cells that lead to the absence of erythrocytosis. The full impact of OSA-induced ROS and inflammation on other organs awaits elucidation. The OSA-mediated blood changes constitute an independent mechanism by which alternations in blood cells contribute to the pathophysiology of OSA. These OSA-driven blood abnormalities may open new avenues for therapy of OSA complications by counteracting increases of ROS and inflammation by antioxidant and anti-inflammatory therapies.
AUTHOR CONTRIBUTIONS
Jihyun Song, conceived the project, performed, designed most of the experiments, critically reviewed and interpreted data, and drafted and finalized the submitted manuscript. Krishna Sundar, conceived the project, recruited OSA patients, designed and supervised the OSA parameters measured, critically interpreted data, and drafted and finalized the submitted manuscript. Monika Horvathova was responsible for the measurements of plasma iron, soluble transferrin receptor, unsaturated iron-binding capacity, ferritin, and hepcidin and edited and approved and finalized the manuscript. Radhika Gangaraju performed chart reviews to abstract clinical data of patients, interpreted data, critically reviewed, edited, and approved the manuscript. Karel Indrak coordinated the laboratory examination of patients with OSA in the Czech Republic and edited and approved the manuscript. Robert Christensen, made end-tidal exhaled carbon monoxide monitor available, interpreted ETCOc data, and critically reviewed, edited, and approved and finalized the manuscript. Samuel Genzor diagnosed, treated, and included Czech patients' data with OSA before and after treatment and determination of red cell mas and approved the manuscript. Carsten Lundby trained Krishna M. Sundar, Jihyun Song, and Josef T. Prchal in using Detalo CO rebreathing equipment, provided control data, helped to interpret data, and edited and approved the manuscript. Vladimir Divoky designed experiments; supervised execution and interpretation of all iron experiments, critically interpreted data and edited, approved, and finalized the submitted manuscript. Tomas Ganz designed experiments; performed erythroferrone measurements, critically reviewed and interpreted data, and edited and approved and finalized the manuscript. Josef T. Prchal, conceived the project, designed experiments; supervised and interpreted all experiments, drafted the manuscript, and finalized the submitted manuscript.
FUNDING INFORMATION
This research was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 2T32HL007576-31 from the National Heart, Lung, and Blood Institute (Jihyun Song) and by I01CX001372-01A2 from the VA Merit Review Award (Josef T. Prchal). Radhika Gangaraju was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5T32DK007115 from the National Institute of Diabetes and Digestive and Kidney Diseases, K08 HL159290 from the National Heart, Lung, and Blood Institute and the American Society of Hematology Scholar Award. Monika Horvathova and Vladimir Divoky were supported by European Union—Next Generation EU, Program EXCELES, ID Project No. LX22NPO5102, Ministry of Health of the Czech Republic (NV19-07-00412), and by the Internal grant of Palacky University (IGA_LF_2023_002). Karel Indrak was supported by Ministry of Health of the Czech Republic (FNOL, 00098892). Tomas Ganz was supported from the National Institute of Diabetes and Digestive and Kidney Diseases 1R01DK126680.
CONFLICT OF INTEREST STATEMENT
Krishna M. Sundar has served as consultant for Resmed Inc. from 2019 to 2021. Currently he is on Advisory Board for Merck Inc. (cough therapeutics) and is a cofounder of Hypnoscure LLC (software for population management of sleep apnea; owned by University of Utah Technology Commercialization office; no fiscal reimbursement received). Radhika Gangaraju has served as a consultant for Alexion and Sanofi.
Open Research
DATA AVAILABILITY STATEMENT
Any data or resources in this manuscript will be shared upon the corresponding author on reasonablerequest.




