A phase 1/2 of carfilzomib and melphalan conditioning for autologous stem cell transplantation for multiple myeloma (CARAMEL)
Abstract
In this phase 1/2 study, carfilzomib was added to high-dose melphalan conditioning prior to autologous stem cell transplantation (ASCT) in patients with multiple myeloma that had been treated with ≤2 prior lines of therapy. Carfilzomib was escalated at doses of 27, 36, 45, and 56 mg/m2 on days −6, −5, −2, and −1 before ASCT in the phase 1 component of the study. In addition, all the patients received melphalan 100 mg/m2 on days −4 and −3. The primary endpoint of the phase 1 component was to identify the maximum tolerated dose, and the primary endpoint of the phase 2 component was the rates of complete response (≥CR) at 1 year after ASCT. The phase 1 dose escalation cohort included 14 patients, and 35 patients were included in the phase 2 cohort. The maximum tested dose was 56 mg/m2 (MTD). The median time from diagnosis to study enrollment was 5.8 (range 3.4–88.4) months, and 16% of patients had obtained a ≥CR prior to ASCT. The best response within 1 year after ASCT was a ≥ CR rate in 22% for the entire cohort, and 22% for patients treated at the MTD. The ≥VGPR rates improved from 41% before ASCT to 77% by 1 year after ASCT. One patient had a grade 3 renal adverse event, and renal function returned to baseline with supportive care. The rate of grade 3–4 cardiovascular toxicity was 16%. The addition of carfilzomib to melphalan conditioning was safe and resulted in deep responses after ASCT.
1 INTRODUCTION
Multiple myeloma (MM) is a plasma cell malignancy that accounts for approximately 1% of all cancers. Though it remains incurable, the survival of patients with MM continues to improve, in large part due to the upfront use of novel therapeutic agents1-4 and increased use of intensive treatments including transplantation in older adults.5, 6 Deeper hematologic responses have also been associated with improvements in the progression free survival (PFS)7-9 and overall survival (OS)8-11 of patients with MM, and the depth of response increases after autologous stem cell transplantation.9, 11 The use of autologous stem cell transplantation (ASCT) has been associated with a survival benefit compared to conventional chemotherapy alone,12, 13 and a PFS benefit in the era of novel agents.14 Therefore, despite expansion of the therapeutic armamentarium for induction, maintenance, and treatment of relapsed or refractory MM, ASCT remains the standard of care for fit eligible patients with multiple myeloma.15, 16
High-dose melphalan has been established as the standard of care conditioning regimen, based on a phase-three randomized control trial which showed an OS benefit with melphalan 200 mg/m2 compared with melphalan 140 mg/m2 combined with 8 Gy total body irradiation.17 However, despite the use of novel agent induction and melphalan conditioning, there remains a subset of patients who still do not obtain deep responses after ASCT. In the IFM-2009 trial, patients who received triplet novel-agent induction followed by high-dose melphalan conditioning and ASCT had a complete response rate of only 59%.14
Based on the observed synergy between bortezomib and melphalan in clinical trials,18, 19 bortezomib has been added to high-dose melphalan conditioning in both the newly diagnosed and relapsed MM setting and resulted in deep remissions with tolerable side effects.20-22 Carfilzomib is a second-generation proteosome inhibitor that irreversibly binds to the proteasome and induces a dose-dependent inhibition of proliferation and apoptosis of plasma cells.23 Carfilzomib has demonstrated increased potency when compared to bortezomib in vitro and in some clinical trials.23-25 The addition of carfilzomib to the conditioning regimen has been shown to be effective and safe in patients with relapsed MM with a median of 3 prior lines of therapy.26 Therefore, this study aimed to assess the safety and efficacy of adding carfilzomib to high-dose melphalan conditioning earlier in the disease course.
2 METHODS
2.1 Patient eligibility
Patients were included in the phase 1 and 2 components of the trial if they were at least 18 years of age with a diagnosis of symptomatic myeloma, were eligible for high-dose chemotherapy with full-dose melphalan (200 mg/m2), had an Eastern Cooperative Oncology Group (ECOG) performance score of 2 or less, and had received 2 or fewer previous lines of therapy, a serum creatinine of ≤2 mg/dL, absolute neutrophil count (ANC) ≥1 × 109 per L, platelet count ≥50 × 109 per L, and hemoglobin ≥8 g/dL. Prior proteosome inhibitor exposure, including carfilzomib, was allowed. To be considered for enrollment, patients were required to have measurable disease (defined as serum monoclonal protein ≥1 g/dL, or 24-h urine monoclonal protein ≥200 mg, or bone marrow plasma cells ≥30%, or abnormal free light chain ratio with involved free light chain ≥10 mg/L) at diagnosis, or at the time of relapse prior to study enrollment. Patients were required to have a left ventricular ejection fraction of ≥45%. Exclusion criteria included a history of New York Heart Association class III or IV heart failure, uncontrolled angina, severe uncontrolled ventricular arrythmias, or electrocardiographic evidence of acute ischemia or active conduction system abnormalities. Additional exclusion criteria included the prior receipt of an autologous or allogeneic stem cell transplant, active HIV infection, active hepatitis B or C infection, or inadequate pulmonary reserve (defined as corrected diffusion lung for carbon monoxide of <50%, FEV1 < 50% predicted, or FVC <50% predicted). All the participating patients gave informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
2.2 Study design
This was an investigator sponsored phase 1/2 open label, single-arm protocol (clinical trials.gov NCT 01842308) with patients accrued from the Rochester (Minnesota) and Jacksonville (Florida) Mayo Clinic sites. Patients admitted between June 4, 2013 and October 11, 2017 were enrolled. The protocol was approved by the institutional review board.
The phase 1 component of the study was initiated as a two-stage accelerated design at dose level 0. However, due to limited patient accrual, the study was amended to a standard cohort of 3 design when the dose was escalated to dose level 1. One patient assigned to dose level 1 received dose level 0 in error and was included with dose level 0 for analysis. During the phase 1 dose escalation, patients received intravenous carfilzomib 27 mg/m2 (dose level 0), 36 mg/m2 (dose level 1), 45 mg/m2 (dose level 2), and 56 mg/m2 (dose level 3) on days −6, −5, −2, and −1. Patients with a body surface area (BSA) greater than 2.2 m2 received carfilzomib at a dose based on 2.2 m2 BSA. Patients also received melphalan 100 mg/m2 on days −4 and −3 (Mel200). Autologous stem cells were re-infused on day 0. The MTD of carfilzomib, as determined by the phase 1 component of the study, was expanded and used for the phase 2 component of the study. The phase 2 dosing and schedule of melphalan administration was consistent with phase 1. All the patients received standard supportive care measures, including blood transfusions, growth factor support, and prophylactic or therapeutic antibiotics according to local institutional guidelines at that time. Maintenance therapy was instituted at the investigator's discretion and was not mandated by the trial protocol.
All the patients underwent complete disease staging prior to study enrollment. Disease response assessment was performed at day 100 after stem cell infusion and then every 90 days thereafter until 1 year after transplant or disease progression, whichever occurred earlier. Diagnostic laboratory measurements were used as the reference to assess disease response. If the patients experienced disease progression prior to receiving the conditioning regimen, the laboratory measurements obtained at relapse were used as the baseline values for disease response assessment.
All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Data on adverse events were collected at baseline (within 14 days of registration), after the last dose of carfilzomib administration (day −6), at day +30 after stem cell infusion, and then every 90 days until 1 year or disease progression, whichever occurred first.
2.3 Study endpoints
The primary endpoint of the phase 1 trial component was to assess the maximum tolerated dose (MTD) of carfilzomib to be combined with Mel200. The MTD was defined as the dose level below the lowest dose that resulted in dose-limiting toxicity (DLT) in at least one third of the patients. DLTs for the phase 1 portion of the study included ANC engraftment (ANC >500 1 × 109/L for 3 consecutive days) delayed beyond day 21 after stem cell infusion, platelet engraftment (platelets >20 × 109/L without transfusion for 3 days) delayed beyond day 30 after stem cell infusion, ≥ grade 3 sensory or motor neurologic toxicity, or ≥ grade 4 non-neurologic or non-hematologic toxicities (excluding nausea, vomiting, or diarrhea).
The primary endpoint of the phase 2 trial was to assess the rate of complete responses (CRs) as a best response assessed on two consecutive evaluations. CR was defined as per the 2011 International Myeloma Working Group (IMWG) uniform response criteria.27 Secondary endpoints included assessment of the CR rates at day +100 after ASCT, progression free survival (PFS) at years 1 and 2 after registration, and toxicities associated with the study intervention. All evaluable patients were used to assess disease response, and all patients that received a dose of carfilzomib were evaluated for efficacy endpoints and adverse events.
2.4 Statistical analysis
The data are presented as of February 18, 2021, when all the registered patients had been followed for at least 12 months. The study was designed to enroll a minimum of 10 patients in phase 1, and a maximum of 39 evaluable patients in phase 2. Per study design, phase 1 patients treated at the maximum tested dose were included in the phase 2 portion of the study. The phase 2 portion of this study utilized a one-stage binomial design that used 39 evaluable patients to test the null hypothesis that the true success proportion (complete response rate) in this patient population is at most 35% versus the alternative hypothesis that the true success proportion is at least 55%.
Continuous numerical outcomes are described using the median and range. Time to event analysis was performed using the Kaplan Meier method. PFS was defined as the time from registration to death or disease progression. Overall survival (OS) was defined as the time from registration to death. All statistical analyses were performed using R (version 3.6.2). A two sided p value <.05 was considered statistically significant.
2.5 Role of the funding source
Onyx/Amgen provided financial support to perform the study, as well as access to carfilzomib administered during conditioning. The sponsor did not participate in study design, interpretation of results, or manuscript preparation.
3 RESULTS
A total of 50 patients were accrued between June 4, 2013 and October 11, 2017. One patient withdrew from the study prior to enrollment due to patient preference. Therefore, 49 patients were included in this study, with 14 patients in the phase 1 dose escalation cohort, and 35 patients in the phase 2 cohort. Only 1 patient experienced a dose-limiting toxicity (delayed neutrophil engraftment beyond day +21 after CD34+ stem cell infusion) at the 56 mg/m2 dose level, and therefore, the MTD was not reached, and the maximal tested dose of carfilzomib used for the phase 2 portion of the study was 56 mg/m2. Six patients in the phase 1 arm of the study received the 56 mg/m2 dose of carfilzomib and were therefore grouped with phase 2 patients for the reporting of efficacy and safety results.
The baseline characteristics of both study cohorts are shown in Table 1. The median age of patients enrolled in both the phase 1 and 2 arms of the trial was 60 (range 42–77) years. The median time from diagnosis to study enrollment was 5.8 months (range 3.4–88.4) months. Overall, 21 patients had high-risk cytogenetics at study enrollment (9 with isolated gain1q, 3 with t(4;14), 4 with t(14;16), and 5 with del(17p)). All the patients received either an immunomodulatory drug (IMID) or a proteosome inhibitor during induction therapy. Of the 4 patients who received carfilzomib during induction, none were refractory. At study enrollment, 8 (16%) of patients had achieved a complete response or better. In both cohorts, 43 (88%) patients had achieved a partial response or prior to ASCT (as shown in Figure 1A). Three (6%) patients had progressive disease at study enrollment. They had received triplet (bortezomib, lenalidomide, and dexamethasone) induction, two patients were enrolled on this study at relapse without salvage therapy, and 1 patient was primary refractory and received high-dose cyclophosphamide prior to enrolling on study.
| Phase 1 (n = 8) | Phase 1 MTD + Phase 2 (n = 41) | Total (n = 49) | |
|---|---|---|---|
| Median age at enrollment—years (range) | 60.5 (44–66) | 60.0 (42–77) | 60.0 (42–77) |
| Gender—n (%) | |||
| Female | 1 (12) | 16 (39) | 17 (35) |
| Male | 7 (88) | 25 (61) | 32 (65) |
| Renal function | |||
| CrCl ≥40 mL/min—n (%) | 8 (100) | 40 (98) | 48 (98) |
| CrCl <40 mL/min—n (%) | 0 (0) | 1 (2) | 1 (2) |
| ECOG Performance Status—n (%) | |||
| 0 | 7 (88) | 27 (66) | 34 (69.4%) |
| 1 | 1 (12) | 12 (29) | 13 (26.5%) |
| 2 | 0 (0) | 2 (5) | 2 (4.1%) |
| ISS stage at diagnosis—n (%) | |||
| Missing | 2 | 13 | 15 |
| Stage I | 3 (50) | 8 (29) | 11 (32) |
| Stage II | 2 (33) | 13 (46) | 15 (44) |
| Stage III | 1 (17) | 7 (25) | 8 (24) |
| FISH risk stratification—n (%) | |||
| Standard risk | 5 (63) | 20 (49) | 25 (51) |
| High riska | 3 (38) | 18 (44) | 21 (43) |
| Missing | 1 (13) | 3 (7) | 4 (8) |
| Median time from diagnosis to study enrollment—months (range) | 5.8 (4.5–8.6) | 5.8 (3.4–88.4) | 5.8 (3.4–88.4) |
| Median lines of therapy prior to study enrollment—n (range) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| Induction therapy | |||
| Cyclophosphamide—n | 9 | 4 | 13 |
| IMID containing regimen—n | 8 | 34 | 42 |
| Lenalidomide/ thalidomide/ pomalidomide—n | 8/0/0 | 34/0/0 | 42/0/0 |
| Proteosome inhibitor containing regimen—n | 13 | 35 | 48 |
| Bortezomib/ixazomib / carfilzomib—n | 12/1/0 | 30/1/4 | 42/2/4 |
| Anti-CD38 antibody—n | 0 | 0 | 0 |
| Response to induction therapy at study enrollment—n (%) | |||
| ≥CR | 2 (25) | 6 (15) | 8 (16) |
| ≥VGPR | 3 (38) | 17 (41) | 20 (41) |
| ≥ORR | 8 (100) | 35 (85) | 43 (88) |
| ≤MR | 0 (0) | 6 (15) | 6 (12) |
- Abbreviations: IMID, immunomodulator drug; ORR, defined as a partial response or greater.
- a High risk cytogenetics was defined as the presence of del(17p), t(4;14), t(14;16), t(14;20), or gain1q.
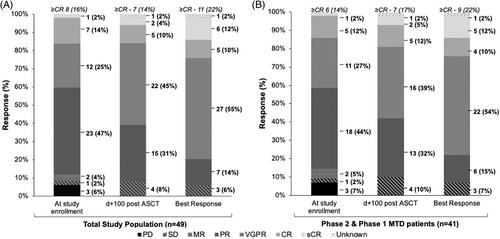
3.1 Efficacy outcomes
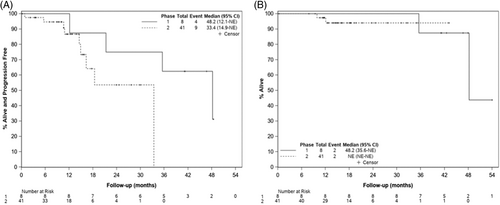
At the time of data cutoff on February 15, 2021, the median follow-up for phase 1 patients was 15 (range 0.3–43.1) months, and 16 (range 0.3–62.3) months for phase 2 patients. One patient was taken off the study due to treatment-related adverse events (TAE) after 1 dose of carfilzomib conditioning and was therefore not evaluable for after ASCT response assessment. A best response of ≥CR was achieved in 9 (22%) patients in the phase 2 arm, and 11 (22%) of the total study population, as shown in Figure 1A,B. At day +100 after ASCT transplant, the ≥CR rates were comparable to the ≥CR rates at study enrollment [d + 100 after ASCT ≥CR was seen in 7 (14%) of patients overall, and 7 (17%) of patients in the phase 2 cohort]. The responses deepened during follow-up. Eight patients, all with high-risk cytogenetics at diagnosis, underwent post-transplant consolidation; 7 patients received triplet regimens, and 1 patient underwent a second autologous stem cell transplantation. In total, 38 patients received maintenance therapy after transplantation (maintenance regimens are outlined in Table S1). The 1-year PFS was 100% (95% CI 100%–100%) for phase 1 patients, and 86.7% (95% CI 67.7%–94.9%) for patients in the phase 2 cohort. The 2-year PFS was 75% (95% CI 31.5%–93.1%) for phase 1 patients, and 53.5% (95% CI 23.7%–76.2%) for patients in the phase 2 cohort (see Figure 2).
3.2 Safety outcomes
In total, 5 patients had carfilzomib doses omitted due to TAE, and 1 patient had omitted doses due to non-TAE (progressive neurological compromise from a known vertebral compression fracture that required emergency surgery). Three patients received only 2 of the 4 scheduled doses of carfilzomib and had subsequent doses omitted due to acute kidney injury (AKI), both patients had resolution of their AKI with supportive measures. One patient received 2 of 4 scheduled carfilzomib doses due to asymptomatic self-limited sinus bradycardia that occurred after carfilzomib infusion. Overall, 3 patients were taken off the study; one patient was taken off the study after 1 dose of carfilzomib developed pulmonary edema in the setting of rapid atrial fibrillation (this patient had a normal left ventricular ejection fraction at the pre-transplant evaluation, but had a known history of poorly controlled atrial fibrillation), 1 patient developed AKI after 2 doses of carfilzomib, and 1 patient was taken off the study for emergent neurosurgery for a vertebral compression fracture causing cord compression. Of the 3 patients taken off the study, 2 patients received melphalan conditioning and therefore included in the outcome and safety assessments, and 1 patient was lost to follow-up.
Within the first 30 days of enrollment, 27 (65.9%) patients in both the phase 1 and 2 cohorts experienced a grade 3–4 non-hematological adverse. In this time period, 36 (87.8%) patients in the phase 2 cohort experienced at least one grade 3–4 adverse event, and 24 (58.5%) patients experienced at least one grade 3–4 non-hematological adverse event. The most common grade 3–4 non-hematologic adverse events that occurred within 30 days of enrollment are shown in Table 2, and most are consistent with expected toxicities during high-dose melphalan ASCT. Within the first 30 days of enrollment, grade 3–4 cardiovascular toxicity was observed in 8 (16%) patients (2 with hypotension that resolved with fluids, 4 with hypertension, 1 with syncope, and 1 with supraventricular tachycardia). One patient was admitted to hospital for grade 3 hypertension and renal failure with a thrombotic microangiopathy on day −4 of conditioning (after receiving 2 doses of carfilzomib 56 mg/m2). The patient was managed supportively with intravenous hydration, anti-hypertensive agents, and n-acetylcysteine and renal function normalized within 10 days. This patient was the only case of grade 3 renal failure in this study. There were no deaths recorded through day +100. Two patients died during the course of study follow-up, both due to progressive disease.
| Phase 1 (n = 8) | Phase 1 MTD + Phase 2 (n = 41) | |||||
|---|---|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grade 4 | Grades 1–2 | Grade 3 | Grade 4 | |
| Nausea | 2 (25%) | 0 | 0 | 7 (17.1%) | 1 (2.4%) | 0 |
| Vomiting | 0 | 0 | 0 | 2 (4.9%) | 1 (2.4%) | 0 |
| Anorexia | 0 | 0 | 0 | 1 (2.4%) | 1 (2.4%) | 0 |
| Diarrhea | 0 | 0 | 0 | 1 (2.4%) | 3 (7.3%) | 0 |
| Esophagitis | 0 | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Mucositis oral | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Dehydration | 0 | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Gastroesophageal reflux | 0 | 0 | 0 | 2 (4.9%) | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Platelet count decreased | 0 | 0 | 8 (100%) | 3 (7.3%) | 0 | 38 (92.7%) |
| Anemia | 3 (37.5%) | 5 (62.5%) | 0 | 8 (19.5%) | 15 (36.6%) | 0 |
| White blood cell decreased | 0 | 0 | 8 (100%) | 0 | 0 | 38 (92.7%) |
| Neutrophil count decreased | 0 | 0 | 4 (50%) | 2 (4.9%) | 0 | 7 (17.1%) |
| Lymphocyte count decreased | 0 | 0 | 3 (37.5%) | 0 | 0 | 8 (19.5%) |
| Infections | 0 | 2 (25%) | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 1 (12.5%) | 0 | 0 | 1 (2.4%) |
| Hypocalcemia | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Hyponatremia | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Hypokalemia | 1 (12.5%) | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Hypophosphatemia | 0 | 1 (12.5%) | 0 | 0 | 6 (14.6%) | 0 |
| Hyperglycemia | 0 | 0 | 0 | 1 (2.4%) | 2 (4.9%) | 0 |
| Hypoalbuminemia | 0 | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Aspartate aminotransferase increased | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Blood bilirubin increased | 0 | 0 | 0 | 1 (2.4%) | 0 | 0 |
| Hypotension | 4 (50%) | 1 (12.5%) | 0 | 16 (39%) | 1 (2.4%) | 0 |
| Hypertension | 0 | 1 (12.5%) | 0 | 0 | 3 (7.3%) | 0 |
| Presyncope | 2 (4.9%) | 0 | 0 | 0 | 0 | 0 |
| Syncope | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Atrial fibrillation | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 |
| Supraventricular tachycardia | 0 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| Creatinine increased | 2 (25%) | 0 | 0 | 8 (20%) | 1 (2.4%) | 0 |
| Peripheral motor neuropathy | 1 (12.5%) | 0 | 0 | 7 (17.1%) | 2 (4.9%) | 0 |
| Peripheral sensory neuropathy | 2 (25%) | 0 | 0 | 8 (19.5%) | 1 (2.4%) | 0 |
| Alopecia | 0 | 0 | 0 | 9 (22%) | 0 | 0 |
| Fatigue | 1 (12.5%) | 0 | 0 | 7 (17.1%) | 2 (4.9%) | 0 |
- a As assessed by study investigators.
The median number of CD34+ cells infused was 5.2 (range 3.1–5.8) x106 cells/kg for phase 1 patients, and 3.8 (range 3.1–7.5) × 106 cells/kg for patients in the phase 2 cohort. The median time to neutrophil engraftment was 15 (range 11–21) days for patients in the phase 1 cohort, and 14 (range 10–24) days for patients in the phase 2 cohort. The median time to platelet engraftment was 10.5 (range 7–13) days for patients in the phase 1 cohort and 12 (range 8–14) days for the phase 2 cohort. The median number of RBC transfusions for phase 1 patients was 1, (range 0–4) and for phase 2, median was 0 (range 0–4). The median number of platelet transfusions was 2, (range 1–4) for phase 1 patients, and 3 (range 0–7) for phase 2 patients. Only one patient received growth factor support to accelerate white cell engraftment.
4 DISCUSSION
This phase 1/2 trial was designed to examine whether adding carfilzomib to full-dose melphalan was safe and could improve the efficacy of the conditioning therapy prior to a planned first single autologous stem cell transplant. To our knowledge, this is the first study to combine carfilzomib as part of the conditioning regimen in the upfront transplant setting. The primary efficacy endpoint was the CR rate as a best response within the first year after ASCT. While the ≥CR rates remained similar (16% before ASCT and 22% as a best response within the first year after ASCT), the ≥VGPR rates increased from 41% before ASCT to 77% within 1 year after ASCT. In contrast, in the transplantation arm of the IFM-2009 study, after 3 cycles of triplet induction with bortezomib, lenalidomide, and dexamethasone, followed by high-dose melphalan ASCT, the ≥VGPR after ASCT was comparable to our study, at 78%.14 The ≥CR rates and PFS were much higher in the IFM-2009 study compared to our study. This may be related to suboptimal maintenance treatment after ASCT given the time period this study was conducted (IFM-2009 mandated lenalidomide maintenance, whereas 22% of the patients in this study did not have maintenance therapy), the inclusion of more patients with conventional high-risk cytogenetics (the proportion of patients with available FISH results that had t(4;14), t(14;16), or del(17p) was 12/45 patients in this study compared to 46/259 patients in the transplantation arm of IFM-2009).14 This study was designed assuming the null hypothesis that the CR rate after ASCT with melphalan alone was 35% (as determined by a retrospective cohort study28). Therefore, given the suboptimal CR rates with the addition of carfilzomib to melphalan conditioning, this regimen may not be more effective than the current standard of care (melphalan alone) if studied in subsequent studies in this patient population.
In 2018, Costa et al. published the first phase 1/2 clinical trial evaluating carfilzomib as part of conditioning in the relapsed/refractory setting, with subsequent maintenance therapy for 12 months after transplant.26 Carfilzomib was administered on days −3 and −2 before ASCT at a dose of 27 and 56 mg/m2, respectively, and then followed by carfilzomib maintenance. The ≥VGPR rates improved from 13.7% before ASCT to 59.2% at 100 days after ASCT in the relapsed refractory MM patient population.26 Our study resulted in higher response rates after ASCT, which may be a result of inclusion of less refractory patients, and higher doses of carfilzomib in the conditioning regimen.
Historical efforts aimed at optimizing the standard conditioning by either increasing the melphalan dose29 or adding additional alkylating agents30-33 has resulted in higher toxicity rates or a lack of improved outcomes. The GEM2000 study showed a significant improvement in PFS with the use of oral busulfan combined with melphalan; however, there were higher rates of transplant-related mortality due to increased veno-occlusive disease (VOD) in patients receiving oral busulfan.34 Given that the use of intravenous busulfan reduces the VOD risk, a single-center phase 3 trial was subsequently randomizing MM patients to conditioning with either busulfan and melphalan combined versus melphalan alone.32 Though the PFS was significantly longer with busulfan and melphalan, the toxicity rates were higher, and there was no difference in OS between arms. The maximal tested dose of our study was 56 mg/m2 and was given at a cumulative dose of 224 mg/m2 (4 doses over 1 week prior to ASCT). Though this study used a fixed dose of carfilzomib over a short duration, the cumulative dose of carfilzomib given over a 1-week span is higher than carfilzomib doses used presently,35-40 owing to the time period in which our study was designed. Our study also lacked pharmacokinetic data to support administration of carfilzomib at this dose. In an early phase 1 study of carfilzomib and dexamethasone, the maximal tested dose of carfilzomib was twice weekly 56 mg/m2 given renal DLT's at a dose of 70 mg/m2.35 Carfilzomib was administered 56 mg/m2 twice weekly in the ENDEAVOR study.39 However, the ARROW study subsequently showed that carfilzomib 70 mg/m2 resulted in a significantly prolonged PFS and similar toxicity compared to a dose of 27 mg/m237 The ENDURANCE and CLARION trials have since used a carfilzomib dose of 36 mg/m2 twice weekly.38, 40
Though our study used higher doses of carfilzomib than current standard of care, this did not result in rates of serious toxicity higher than expected. A systematic review that characterized rates of renal toxicity in randomized trials comparing carfilzomib with non-carfilzomib regimens found that in the 4 included trials, the cumulative rate of all-grade renal toxicity was 8.3%, and grade 3–5 renal toxicity occurred in 21.3% of the patients.41 Similar rates of renal dysfunction have been reported in retrospective cohort studies, and renal failure has been shown to be reversible in approximately 60% of the patients.42 Only 1 patient (2%) had grade 3–4 renal toxicity, and renal function returned to baseline with supportive care. Furthermore, though carfilzomib cardiovascular toxicity may be dose-dependent,43 grade 3–4 cardiovascular toxicity was seen in 16% of our study participants. Cardiovascular toxicity related to carfilzomib has been well described and can present as hypertension, arrythmias, ischemic events, or heart failure with reduced ejection fraction. In a systematic review of 24 prospective studies of carfilzomib, the reported rates of grade ≥3 cardiovascular adverse events was 8.2%.43
In conclusion, our study showed that the addition of carfilzomib to high-dose melphalan conditioning did not increase the rate of CR's, but did improve the proportion of patients achieving a ≥VGPR. Despite the high doses of carfilzomib administered in the study, the extent of renal and cardiovascular toxicity was similar to previously reported carfilzomib-containing regimens.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant conflicts of interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Anonymized data are available from the corresponding author upon request.




