Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management
Abstract
Disease overview
The myelodysplastic syndromes (MDS) are a very heterogeneous group of myeloid disorders characterized by peripheral blood cytopenias and increased risk of transformation to acute myelogenous leukemia (AML). MDS occurs more frequently in older males and in individuals with prior exposure to cytotoxic therapy.
Diagnosis
Diagnosis of MDS is based on morphological evidence of dysplasia upon visual examination of a bone marrow aspirate and biopsy. Information obtained from additional studies such as karyotype, flow cytometry, and molecular genetics is usually complementary and may help refine diagnosis. A new WHO classification of MDS was proposed in 2022. Under this classification, MDS is now termed myelodysplastic neoplasms.
Risk-stratification
Prognosis of patients with MDS can be calculated using a number of scoring systems. All these scoring systems include analysis of peripheral cytopenias, percentage of blasts in the bone marrow, and cytogenetic characteristics. The most commonly accepted system is the Revised International Prognostic Scoring System (IPSS-R). Recently, genomic data has been incorporated resulting in the new IPSS-M classification.
Risk-adapted therapy
Therapy is selected based on risk, transfusion needs, percent of bone marrow blasts, cytogenetic and mutational profiles, comorbidities, potential for allogeneic stem cell transplantation (alloSCT), and prior exposure to hypomethylating agents (HMA). Goals of therapy are different in lower risk patients than in higher risk and in those with HMA failure. In lower risk, the goal is to decrease transfusion needs and transformation to higher risk disease or AML, as well as to improve survival. In higher risk, the goal is to prolong survival. In 2020, two agents were approved in the US for patients with MDS: luspatercept and oral decitabine/cedazuridine. In addition, currently other available therapies include growth factors, lenalidomide, HMAs, intensive chemotherapy, and alloSCT. A number of phase 3 combinations studies have been completed or are ongoing at the time of this report. At the present time there are no approved interventions for patients with progressive or refractory disease particularly after HMA based therapy. In 2021, several reports indicated improved outcomes with alloSCT in MDS as well as early results from clinical trials using targeted intervention.
1 DISEASE OVERVIEW
Myelodysplastic syndrome (MDS) comprises a very heterogeneous group of myeloid malignancies with very distinct natural histories.1 MDS occurs in 3–4 individuals per 105 in the US population.2 Prevalence increases with age. In individuals age 60 and above, prevalence is 7–35 per 105.2 Other series have reported higher rates.3 MDS affects more frequently males than females.2 Exposure to prior chemo or radiation therapy is a risk for the development of MDS. Work over the last two decades has demonstrated that MDS is an heterogenous group of malignancies arising from distorted hematopoietic stem cell function,4 inflammatory and innate immune deregulation,5 deregulated apoptosis,6 and multiple genomic events.7 This combination of molecular alterations results anemia, increased risk of infections, bleeding, and transformation to acute myelogenous leukemia (AML).8 A majority of patients with MDS will die from complications of MDS without transforming to AML and therefore the need for unique treatment strategies for patients with MDS.8 Further adding importance to this concept is the discovery of the relation between comorbidities and clonal hematopoiesis9 and MDS10 suggesting an interplay between MDS and the development of other conditions such as cardiovascular disease.11
MDS is usually suspected by the presence of cytopenia on a routine analysis of peripheral blood. This prompts evaluation of bone marrow cell morphology (aspirate) and cellularity (biopsy). A manual count of bone marrow blasts is fundamental for risk assessment. Cytogenetic analysis helps in predicting risk and in selecting therapy. Once this information is collected, MDS risk can be calculated. Patients with MDS can be stratified according to several internationally accepted scoring systems. The original IPSS12 and the modified IPSS-R13 are the most commonly used systems. These two systems are also important because they serve as part of the main eligibility criteria for past and ongoing registration clinical trials.14 Using IPSS and IPSS-R, patients with MDS are generally divided in two different broad subgroups: lower and higher risk disease. Patients with lower risk disease by IPSS are those with low and intermediate-1 disease and very, low and some subsets of intermediate risk by IPSS-R. Patients with higher risk disease are those with intermediate-2 and high risk by IPSS and some subsets of intermediate, high, and very high-risk disease by IPSS-R. Because the IPSS-R divides patients into five subsets, there is some controversy in terms of what subset of patients with IPSS-R intermediate disease should be considered lower or higher risk disease.15 Recently, molecular data has been incorporated to calculate prognosis in MDS resulting in the new IPSS-M classification.16 Several other important data points are needed when making treatment decisions in patients with MDS. These include age of the patient, type and severity of comorbidity,17 significance and number of cytopenias, transfusion needs, presence of specific genomic alterations (now computed by the IPSS-M),16 percentage of blasts, cytogenetic profile, potential for allogeneic stem cell transplantation (alloSCT), and importantly prior treatment with a hypomethylating agent (HMA). This is fundamental as the biology and natural history of patients with MDS that have been treated with an HMA is very different from that of patients not previously treated with such an agent with similar IPSS and IPSS-R scoring systems.18, 19
2 DIAGNOSIS
The diagnosis of MDS is generally suspected based on the presence of cytopenia. Diagnosis is confirmed by performing a bone marrow aspiration and biopsy. Both procedures provide different information. The bone marrow aspirate allows for detailed evaluation of cellular morphology and evaluation of percent of blasts. The bone marrow biopsy allows for determination of bone marrow cellularity and architecture. Diagnosis is established by the presence of dysplasia. A number of morphological classifications are in place. The most recent one being the 2022 WHO classification (Table 1).20 Under this classification, MDS is now myelodysplastic neoplasm, acronym MDS. I believe changing the name is a mistake as these disorders are clearly syndromic not just neoplasms. This is for instance demonstrated by its associations with comorbities.10 In addition, a parallel effort was proposed by the ICC.21 My opinion is that the percentage of blasts in MDS should be still considered up to 20%. Decreasing the percentage of blasts to 10% because prognosis is similar when compared to patients with AML makes no scientific sense as prognosis does not equate diagnosis. Using this paradigm, we could describe all diseases, solid and liquid tumors, with a poor risk abnormality, that is, p53 mutation, with the same name just because prognosis is poor or we can target it (i.e., IDH mutations in AML and glioblastoma). In addition, morphologically MDS is different than AML. Details of these classifications have been reviewed elsewhere.
| MDS with defining genetic abnormalities | Blasts |
|---|---|
| MDS with low blasts and isolated 5q deletion (MDS-5q) | <5% BM and <2% PB |
| MDS with low blasts and SF3B1 mutation (MDS-SF3B1) | <5% BM and <2% PB |
| MDS with biallelic TP53 inactivation (MDS-biTP53) | <20% BM and PB |
| MDS, morphologically defined | |
| MDS with low blasts (MDS-LB) | |
| MDS, hypoplastic (MDS-h) | |
| MDS with increased blasts (MDS-IB) | |
| MDS-IB1 | 5%–9% BM or 2%–4% PB |
| MDS-IB2 | 10%–19% BM; or 2%–19% PB |
| MDS with fibrosis | 5%–19% BM; or 2%–19% PB |
A number of additional tests are needed to complete the laboratory evaluation of a patient with MDS. It is well established that cytogenetic patterns are very heterogeneous in MDS.22 Cytogenetics are of importance to calculate prognosis of patients and in some subsets of patients to select the most effective form of therapy. The most recent cytogenetic risk classification in MDS includes 5 different subgroups including 20 different alterations (Figure 1).23 Cytogenetic patterns are not stable in MDS and a significant fraction of patients can acquire additional cytogenetic changes with disease evolution. This phenomenon is associated with increased risk of transformation to AML and worse survival.24 New techniques to assess large structural chromosomal changes, such optical genome mapping, have been reported to be able to perform cytogenetic analysis without the need to culture marrow cells or band interpretation. This results in lower percentage of failed assays.25 These technologies are moving into CLIA hematopathology laboratories.
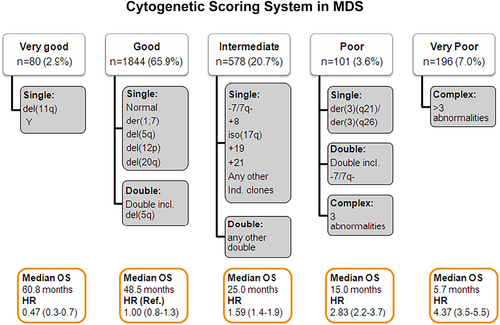
A number of other assays can be used to help in the diagnosis of MDS. These include flow cytometry, fluorescent in situ hybridization (FISH) and genomic sequencing techniques. Flow cytometry can help in the identification of abnormal phenotypic patterns and can be of help in cases of minimal dysplasia. Because the significant heterogeneity of cytogenetic alterations in MDS, there is no evidence that a panel of FISH probes could replace routine 20 metaphase cytogenetic analysis. Thus, in our opinion FISH and flow cytometry should not be considered part of the standard work up evaluation procedure of the patient with MDS and should be used in specific situations. Thanks to the discovery of multiple genetic mutations in MDS, we now have access to clinical tools for molecular annotation of patients with MDS. These can be of use to complement other diagnostic tools. A majority of MDS patients, will carry at least one or more somatic mutations in genes involved in DNA methylation, chromatin regulation, RNA splicing, transcription regulation, DNA repair, cohesin function, or signal transduction.7, 26
2.1 ICUS, clonal hematopoiesis of indetermined potential, and CCUS
Because diagnosis of MDS is based on morphological assessment, it can be subjective particularly in patients with early low risk disease and minimal dysplasia. It is calculated that diagnostic discrepancy can occur at the time of initial presentation in close to 20% of patients.27 This has obvious implications for therapeutic decision making and patient counseling. In general, diagnosis is obvious in patients with excess blasts. The problem is in patients without excess blasts were diagnosis is based on dysplasia. Clinical assessment is needed in patients with minimal or not diagnostic evidence of dysplasia. In these cases, it is recommended that other causes of cytopenia be excluded. Routine tests include the analysis of anemia and thrombocytopenia, and exclusion of cause of blood loss or inflammatory processes. When suspected, evaluation of GI tract needs to be considered. Once other potential causes of cytopenia are excluded, additional diagnostic tools including cytogenetic evaluation, flow cytometry and, more recently, DNA sequencing, can help define the diagnosis and predict patient outcomes. Patients with cytopenia but no dysplasia are considered to have idiopathic cytopenia of undetermined significance (ICUS). A fraction of these patients may have cytogenetic abnormalities or somatic mutations in genes recurrently mutated in myeloid malignancies.28, 29 The presence of a somatic mutation in the context of cytopenias with no diagnostic criteria for MDS is now considered a clonal cytopenia of undetermined significance (CCUS).30 This distinction is supported by data which suggests that, although close to 25% of patients with ICUS may ultimately develop MDS or AML, this risk significantly increases in the presence of a clonal mutation from 9% to 82% at 5 years, particularly in the presence of highly predictive mutation patterns.28, 31 A detailed evaluation and careful differential diagnosis between ICUS, CCUS, and MDS is therefore essential (Table 2). The presence of clonal somatic mutations have also been reported in hematopoietic cells from older individuals without evidence of hematological disorder.32, 33 This is considered as age-related clonal hematopoiesis of indetermined potential (CHIP).30 Clonal hematopoiesis has also been identified as a risk factor for therapy-related MDS and AML.34 Patients with evidence of clonal hematopoiesis are also at increased risk to develop MDS.33 There is significant interest in the evaluation and potential treatment of patients with CHIP or CCUS. This stands from the relationship between other systemic disorders, for instance cardiovascular disease, and CHIP/CCUS and the hypothesis that by treating CHIP/CCUS we could prevent progression to myeloid neoplasm (MN). A number of centers are developing “CHIP clinics” and clinical trials. Potential targets include IL-1, splicing alterations, and IDH mutations among others. Recently, Weeks et al. have proposed a prognostic model of CHIP/CCUS. In addition, data presented at ASH 2022 indicated that the most frequent mutation in CHIP/CCUS is DNMT3A. This is associated with a relative low frequency of progression to MN.35 This data may allow proper selection of individuals with CHIP/CCUS for therapy or more intensive interventions.
| Features | CHIP | ICUS | CCUS | MDS |
|---|---|---|---|---|
| Cytopenia | No | Yes | Yes | Yes |
| Dysplasia | No | No or minimal (nondiagnostic for MDS) | No or minimal (nondiagnostic for MDS) | Yes |
| Somatic mutations | Yes at a variant allele frequency ≥2%. Most commonly: DNMT3A, TET2, ASXL1 | No. ICUS defined by absence of clonality |
|
Yes. Up to 85% of patients |
| Risk of progression | Very low (0.5%–1% per year) outside of therapy related setting. | Very low | Up to 80% at 5 years but determined by mutational patterns. | - |
- Abbreviations: CCUS, clonal cytopenia of undetermined significance; CHIP, clonal hematopoiesis of indeterminate potential; ICUS, idiopathic cytopenia of undetermined significance; MDS, myelodysplastic syndromes.
A subset of patients of importance are those with MDS/MPN features. These are patients with evidence of a myeloproliferative component (with or without fibrosis). At the present time, with the exception of chronic myelomonocytic leukemia, we do not fully understand the natural history of patients with MDS/MPN.36 In our center, they are currently treated as MDS but studies are ongoing to clarify this issue. Of note, specific MDS/MPN subtypes have particular mutational profiles and may benefit from specific therapeutic approaches. An example includes patients with MDS/MPN with ring sideroblasts and thrombocytosis who typically have mutations in SF3B1 and JAK237, 38 and may have good response to therapy with lenalidomide.39
3 RISK STRATIFICATION
The prognosis of patients with MDS is very heterogeneous and thus the need to develop prognostic systems that allow risk stratification and help in the timing and choice of therapy. Apart from the intrinsic prognostic value of morphological classifications,40 a number of prognostic scores are currently in use in MDS. The IPSS12 has been in place since 1997. This score has been discussed in prior versions of this manuscript.41 It has been replaced by en-large by the IPSS-R.13 The IPSS-R includes different cut off points of cytopenias when compared to IPSS and incorporates a more comprehensive cytogenetic score.13 IPSS-R is now the standard tool to assess risk. The IPSS-M was recently published and is has been validated by a number of centers.16, 42 This analysis was developed using data from 2957 patients from 24 centers. The model includes detailed information regarding p53 (mono versus biallelic mutations) and incorporates 16 genes. This tool has not been tested yet in specific subsets of patients such as those treated with hypomethylating agents or HMA failure.
It is also apparent that the natural history of patients with lower risk disease is very heterogeneous.43 We evaluated outcomes in a large series of patients with low or int-1 disease by IPSS. We found that prognosis varied significantly in patients with lower risk MDS and were able to develop a lower risk MDS specific prognostic score.43 This has significant implications for the development of specific interventions for patients with lower risk disease. This model has been validated on several occasions and it is being used to identify patients with poor prognosis lower risk disease that could be candidates for early intervention. Bejar et al. published data indicating that patients with poorer prognosis and lower risk disease accumulate a higher number of mutational events than the better risk counterpart.44 This data provides a potential molecular basis for the identification of this group of patients and poorer prognosis. The new IPSS-M should really help clarify the prognosis in patients with lower risk features such as diploid cytogenetics and low percentage of blasts.
MDS occurs in older patients that suffer from comorbidities. None of the systems discussed above include impact of comorbidity in the calculation of the natural history of MDS patients. To study this issue, we used a comprehensive comorbidity score known as ACE-27 in a cohort of 500 patients with MDS.17 Presence of comorbidity had a significant independent impact on survival and a prognostic score could be developed that included age, IPSS, and ACE-27 score. The same results were obtained using IPSS-R.45 This data indicates the need to add comorbidity scores in MDS. Other groups have confirmed the importance of comorbidity scores in MDS.46
Additional prognostic scoring systems include systems for hypocellular MDS47 and for therapy related MDS.48 However, conventional risk stratification models such as IPSS-R, MDACC model, or the WPSS seem equally valid in therapy related disease.49 It should be noted that prognosis in t-MDS is strongly associated with presence of cytogenetic alterations: diploid patients have prognosis not dissimilar to that of de novo patients.48, 50
4 CYTOGENETIC AND MOLECULAR ALTERATIONS
Over the last decade, a number of very important studies have been published describing comprehensive analysis of the incidence and clinical impact of multiple genetic lesions in MDS.51 Bejar et al. first published an analysis of 18 genes using different techniques on 439 patients.52 Subsequently, two major studies described the mutational landscape of MDS in larger series analyzing more genes.7, 26 An European consortium reported an analysis of mutations in 111 genes using next generation sequencing technology in a cohort of 738 patients.7 Frequency of common mutations is shown in Table 3. Since then, multiple other studies describing the mutational landscape of MDS and its potential prognostic and therapeutic implications have been published. Despite the heterogeneity of some of these studies, mutations in genes such as RUNX1,7, 26, 44 TP53,7, 26, 44, 53, 54 or EZH226, 44 have consistently been associated with adverse prognosis while mutations in the splicing factor SF3B1 are associated with favorable outcomes and prolonged survival.7, 44, 55
| Gene | % | Location | Function |
|---|---|---|---|
| SF3B1 | 28 | 2q33 | Splicing factor |
| TET2 | 21 | 4q24 | Control of cytosine hydroxymethylation |
| ASXL1 | 14 | 20q11 | Epigenetic regulator |
| SRSF2 | 12 | 17q25 | Splicing factor |
| RUNX1 | 9 | 21q22 | Transcription factor |
| TP53 | 8 | 17p13 | Transcription factor |
| U2AF1 | 7 | 21q22 | Splicing factor |
| EZH2 | 6 | 7q36 | Polycomb group protein |
| NRAS | 4 | 1p13 | Signal transduction |
| JAK2 | 3 | 9p24 | Tyrosine kinase |
| ETV6 | 3 | 12p13 | Transcription factor |
| CBL | 2 | 11q23 | Signal transduction |
| IDH2 | 2 | 15q26 | Cell metabolism, epigenetic regulation |
| NPM1 | 2 | 5q35 | Phosphoprotein |
| IDH1 | 1 | 2q33 | As IDH1 |
| KRAS | <1 | 12p12 | Signal transduction |
| GNAS | <1 | 20q13 | G protein |
| PTPN11 | <1 | 12q24 | Protein phosphatase |
| BRAF | <1 | 7q34 | Raf kinase |
| PTEN | <1 | 10q23 | Phosphatase |
| CDKN2A | <1 | 9q121 | Cell cycle control |
5 RISK ADAPTED THERAPY
5.1 Current conceptual framework for the therapy of MDS in 2023
We divide patients into six different categories. First is the subset of patients without morphological diagnosis of MDS that include ICUS, CHIP, and CCUS.56 Next, we divide patients with MDS into lower or higher risk but further subdivided them based on whether they have been exposed to an HMA: lower risk MDS, lower risk HMA failure, higher risk and higher risk HMA failure.18 Finally, a group of patients with extremely poor prognosis is that of patients with AML evolving from MDS particularly after HMA-based therapy. We are not going to discuss this last subset of patients on this review. In addition, clinical grade genomic is aiding in treatment decisions for all these subsets of patients. We discuss these five subsets of patients below. Figure 2 summarizes current treatment strategies for patients with MDS.
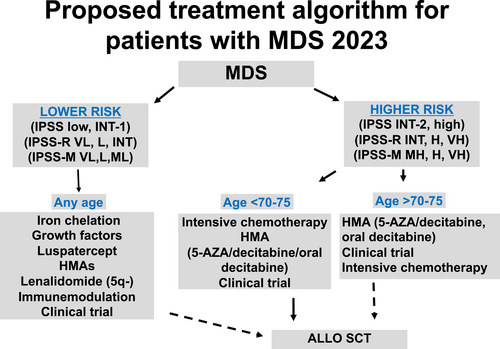
5.2 Treatment of ICUS/CHIP/CCUS
The field of ICUS/CHIP/CCUS was transformed by pioneer work led by Ebert and colleagues. Better understanding of these entities has transformed our understanding of MDS and our approach to these group of patients.33 Several important manuscripts have defined and contextualized ICUS/CHIP/CCUS a topic beyond this review.56 Today there is no data to support treatment of patients with ICUS/CHIP/CCUS. Individuals with ICUS have very low risk of progressing to MN. These individuals could be followed in the community. Patients with CHIP and CCUS have molecular alterations, and in particular in the context of cytopenia (CCUS), should be followed more frequently. A number of institutions are developing “CHIP clinics” to follow such patients and develop guidelines of care. In our group, these patients are followed with peripheral blood analysis every 6–12 months based on degree of cytopenia and type of mutation. Another important finding associated with CHIP/CCUS is not only the increased risk of transformation to MN but also the collateral risk of associated comorbidities.11 Therapies directed toward those comorbidities, that is, cardiovascular disease, are warranted and should be monitored in clinical trials.
One very important subset of patients with CHIP/CCUS is the group of patients that have received prior therapy for another malignancy. Work by Wong et al showed that in patients with therapy-related neoplasms (T-MN) with a p53 mutation, the mutation could be detected in bone marrow specimens obtained prior to the patient ever receiving any therapy for their primary malignancy.57 This finding is important as it suggests that chemotherapy or radiation therapy used to treat primary malignancy may not cause DNA damage per se but expansion of clonally mutated hematopoietic cells that expand with time. Based on this concept, Takahashi and Padron et al in two independent but parallel studies demonstrated that CHIP/CCUS in patients treated for another malignancy are at a very high risk of progression to T-MN.34, 58 Of interest, the data from these two investigators indicated that this phenomenon was not restricted to p53 but can be observed with any other gene mutated in CHIP/CCUS. Also of importance is the observation from a study at MD Anderson that patients with lymphoproliferative process or multiple myeloma treated with an autologous stem cell transplant are at particular risk of developing therapy related neoplasm.48
Recommendation: In 2023, patients with ICUS/CHIP/CCUS should not be treated but followed preferentially in a dedicated “CHIP” clinic. Attention should be paid to the care of comorbidities. Clinical trials could be considered if available.
5.3 Options for patients with lower risk MDS
Therapy in this subset of patients is based on transfusion needs. In general, patients that are transfusion independent are usually observed until they become transfusion dependent. This concept is currently being challenged (see below). Below is a summary of agents currently available for patients with lower risk MDS.
Erythroid growth factor support: The use of erythroid stimulating agents (ESA) is common practice.59 A number of ESAs are available. Response rates range from 30% to 60% depending on study.60 Data from the Swedish group indicated that addition of G-CSF to erythropoietin increased responses rates. In a retrospective observational study, early introduction of this combination in patients with low risk disease and minimally transfusion dependent patients had an impact on survival.61 The Swedish group also developed an algorithm to predict response to ESA.62 The French group also evaluated the impact of ESA on survival in a retrospective study of 284 patients and compared it to a group of patients that formed the IPSS cohort.63 In this study patients treated with ESA had a better survival (HR for death was 0.43, 95% CI 0.25–0.72).63 Results from a recently published randomized placebo-controlled phase 3 study of darbepoetin alfa for 147 patients with lower-risk MDS showed significantly higher erythroid responses (14.7% vs. 0%, p = .016) and reduction in transfusion incidence in weeks 5–24 of therapy (36% vs. 59.2%, p = .008) with darbepoetin compared to placebo, with no significant differences between groups in the incidence of thromboembolic events, solid malignancies or transformation to AML.64 However, questions on potential tumorigenic effect of these drugs has resulted in increased scrutiny of their use. G-CSF is not approved by the FDA for patients with anemia of MDS in the US.
Recommendation: A course of ESA with or without G-CSF is not contraindicated in most patients with low risk MDS with significant anemia without other cytopenia. Data indicates that early incorporation of these agents is more effective. We maintain therapy for at least 3 months to judge efficacy. In responding patients, we continue therapy until transfusion effect is lost. These recommendations may change once we have access to the results of the COMMANDS trial (see below).
Luspatercept: The Medalist trial was reported in 2020.65 This was a multicenter randomized trial of luspatercept for patients with red cell transfusion dependent RARS that were not benefiting from ESAs or were unlikely to benefit.65 Luspatercept modulates TGF-β signaling in MDS resulting in improved erythropoiesis.66 This concept was initially tested in preclinical mouse models and in phase 1 and 2 trials with luspatercept and the related compound sotarcept.67 Two hundred twenty-nine were randomized on the trial. One hundred fifty-three to luspatercept and 76 to placebo. The primary end point of the study was red blood cell (RBC) transfusion independency (TI) for more than 8 weeks following IWG06 criteria.68 Luspatercept resulted in an increase rate of TI in this subset of patients resistant to ESA. TI was achieved in 37.9% of patients with luspatercept versus 13.2 in control (p < .001). At more than 12 weeks, TI was documented in 33.3% of patients on luspatercept versus 11.8% in control. Median duration of RBC TI was 30.6 weeks versus 13.6. Other endpoints such as hematological response-erythroid were also in favor of luspatercept. Toxicity of this compound that is injected subcutaneously every 3 weeks was acceptable without drug related mortality or increased risk of transformation to AML. Based on this data, the US FDA approved luspatercept in 2020.65 Following the results of the Medalist trial, the COMMANDS trial (NCT03682536) was designed to compare luspatercept versus an ESA in transfusion dependent patients with lower risk MDS. This study has been completed and results are expected in 2023.
Recommendation: Luspatercept is the standard of care of patients with RARS that have not responded, lost response or are not candidates to an ESA.65
Lenalidomide: Lenalidomide is approved for patients with lower risk MDS, anemia and alteration of chromosome 5.69 Phase I results70 were confirmed in a subsequent phase II study of lenalidomide in patients with anemia and alteration of chromosome 5.70 In that study 148 patients received 10 mg of lenalidomide for 21 days every 4 weeks or daily. Of those, 112 had decrease need for transfusions (76%; 95% CI 68–82) and 99 patients (67%; 95% CI, 59–74) became transfusion independent. Response was fast: median time 4.6 weeks. The median rise of hemoglobin was 5.4 g/dL. Of interest, cytogenetic responses were observed in close to 50% informative patients. Predictors of response included presence of a platelet count of 100 × 109/L and less than 4 prior units of red cells transfused. It should be noted that in this study patients with a platelet count of less than 50 × 109/L were excluded. Subsequently, these results were confirmed in a phase III known as AZA-004 comparing two different doses of lenalidomide (5 and 10 mg orally daily) versus placebo. This study was also designed to clarify the issue of transformation to AML. 10 mg daily was the superior arm and that there was no increased incidence of transformation to AML in patients treated with lenalidomide.71 Of importance, a report from the initial MDS-003 trial of lenalidomide indicated a longer survival for patients responding to therapy.72
Identifying patients at risk of treatment failure or transformation remains an essential aspect of management of 5q-MDS. Age <65 years,73 bone marrow blast count >5%74, 75 and transfusion dependence have been associated with AML transformation.74 Several studies have suggested the role of TP53 mutations and karyotypic complexity in disease progression and outcome.75-80 Mutations in TP53 can be detected at early stages prior to therapy in 12%–17% of these patients,76-78, 80 and TP53-mutant clones can emerge and expand through the course of lenalidomide therapy and at the time of disease progression.77, 79 Various studies have reported lower response rates and lower likelihood of complete cytogenetic response in TP53-mutated compared with wildtype patients when treated with lenalidomide.76, 78, 80 However, an analysis from a multicenter study including 67 patients with 5q-MDS treated with lenalidomide did not confirm differences in TI rates or complete cytogenetic response based on TP53 mutational status.77 A trend to shorter response duration (6 vs. 16 cycles, p = NS) as well as higher risk of transformation and shorter event-free and overall survival were observed among patients with TP53-mutated 5q-MDS.
Although current data supports initiating lenalidomide at the time of transfusion dependence, data from a subset analysis from the multicenter RevMDS study including 381 patients treated with 10 mg daily of lenalidomide suggested that early intervention in transfusion independent patients with Hgb <10 g/dL may be associated with erythroid responses. In this study, among 12 TI patients with Hgb <10 g/dL therapy with lenalidomide was associated with universal erythroid responses and improved QoL scores compared to pretreatment values (+12.5; p = .02).81 Following this lead, one of the most interesting contributions to field of MDS was presented at ASH 2023 by Diez-Campelo et al: the Sintra-Rev trial.82 In this study, European investigators randomized 60 transfusion independent patients with del5q-MDS to 5 mg lenalidomide or placebo and measured the transfusion free survival (TFS) in each arm. TFS was not reached in patients on the low dose lenalidomide arm versus less than 1 year for those on the placebo (p .003, HR 0.3, p .005). Furthermore, cytogenetic responses were documented in 94% of the lenalidomide patients versus 0% in the placebo. Uncensored data analysis did not show improvement of median survival (8.4 vs. 7.4 years) but it is possible that with more time these curves could separate. Of importance, there was no evidence of increased risk of transformation in patients with p53 mutated disease. I believe this data is of importance as is one of the first examples of successful early intervention in MDS.
Lenalidomide has been investigated in patients without chromosome 5 alterations.83 In an initial study, 214 patients received lenalidomide 10 mg orally daily or 10 mg on days 1–21 of a 28-day cycle. Fifty six (26%) patients achieved transfusion independence after a median of 4.8 weeks of therapy. Median response duration was 41 weeks. An international phase III, randomized, placebo-controlled study including 239 transfusion-dependent lower-risk non-del5q MDS patients confirmed the efficacy of lenalidomide in this setting.84 Patients receiving lenalidomide 10 mg daily for 21 days where significantly more likely to achieve RBC transfusion independence for ≥8 weeks compared to placebo (26.9% vs. 2.5%, p < .001). Median duration of TI was 30.9 weeks and TI was associated with significant improvement in HRQoL scores. In addition, higher response rates were observed in patients with baseline endogenous EPO ≤500 mU/mL. Of note, a recent study not only reported the negative impact of TP53 mutations in response outcomes to lenalidomide in del5q MDS, but also suggested U2AF1 mutations may be likewise associated with a lower likelihood of response both in del5q and non-del5q MDS and that mutations in DEAD box RNA helicase genes (DDX41, DDX54, and DHX29) may be enriched among responders in non-del5q MDS.85 However, these findings have to be confirmed on larger studies.
Recommendation: The degree of response with lenalidomide in patients with lower risk MDS, anemia, good platelets and del5q makes it the standard of care for this subset of patients. This is further reinforced by the data on survival in responding patients.72 We do not consider this agent in patients with thrombocytopenia. Based on the results of the phase III randomized study, lenalidomide can be considered an option for selected red cell-transfusion-dependent patients with non-del5q MDS. Evaluation of TP53 mutation status could be advisable in patients with del5q MDS prior to starting therapy with lenalidomide. Although current evidence does not support withholding therapy with lenalidomide in patients with TP53 mutations, these patients should be monitored closely for signs of inadequate response, loss of response or progression. The final report of the Sintra-Rev trial could also support the use of low dose lenalidomide in del5q-MDS transfusion independent patients.
Azanucleosides: Three azanuclesoides are approved for MDS: 5-azacitidine86 and 5-aza-2′-deoxycitidine (decitabine)87 and in 2020 oral decitabine/cedazuridine (oral dac/ced, ASTX727).88 5-azacitidine is approved for all subsets of MDS whereas decitabine for those with INT-1 disease and above. Oral dac/ced has the same indication than that of decitabine and is described below.
Although these agents are not approved in the European Union for patients with lower risk MDS, they are commonly used in the US. Currently, main use is for patients with multilinear cytopenia or as second line therapy. The main question in the US is the dose and schedule of this class of compound in lower risk MDS. Work from the original studies with oral azacytidine (now CC-486) indicated that lower exposures, as determined by pharmacokinetic profiling, resulted in significant clinical activity.89, 90 Based on this information, at MD Anderson we designed a series of clinical trials investigating attenuated doses and schedules of both azacytidine and decitabine. These are summarized in Table 4. The first one was a randomized trial of decitabine on a daily scheduled × 3 days every 28 days compared to a weekly × 3 also every 28 days.91 Although no differences were observed between both arms, the ORR was 23% and drug related mortality was 0%. Median overall survival was not reached. 70% of patients were alive at 500 days. This study was followed by a randomized study comparing the 3-day decitabine schedule at a dose of 20 mg/m2 versus azacytidine at a dose of 75 mg/m2 also for 3 days.92 From this data, it appeared that the attenuated schedule of decitabine was more effective than that of azacytidine. This study was criticized because potentially it was not comparing equivalent doses of the two HMAs. Based on this and to further study the activity of these two drugs, a third study was designed by the US MDS consortium comparing 2 schedules of azacytidine (3 and 5 days) and one of decitabine (3 days) in both transfusion dependent and independent patients. This study has completed planned accrual. The cumulative data from these studies and other trials from Cleveland Clinic using attenuated low dose chronic schedules of decitabine93 support the use of lower doses of HMAs in lower risk MDS. Indeed, the NCCN guidelines support this concept.14
| Study | N | ORR % | CR % | TI % | OS |
|---|---|---|---|---|---|
| Low dose DAC12 | |||||
| DAC daily x3 | 43 | 23 | 16 | 67 | Not reached |
| DAC weekly x3 | 22 | 23 | 0 | 59 | Not reached |
| DAC versus AZA41 | |||||
| DAC daily x 3 | 73 | 70 | 37 | 32 | Not reached |
| AZA daily x 3 | 40 | 49 | 36 | 16 | Not reached |
| CC-486 versus placebo | |||||
| CC-486 | 107 | NA | NA | 30.8 | 17.3 months |
| Placebo | 109 | 11.1 | 16.2 months |
- Note: Results from 3 randomized trials of attenuated HMA dosing in lower risk MDS. CC-486 was presented at EHA 2020 and has not been formally published.
- Abbreviations: AZA, azacitidine; CR, complete remission; DAC, decitabine; NA, not available; ORR, overall response rate; OS, overall survival; TI, transfusion independency.
Different schedules of 5-azacitidine have been explored in MDS. In a community study a 5 day schedule was compared to a 7 day 5-2-2 schedule (weekend off) or a 5-2-5 schedule of 10 days.94 Fifty patients were assigned to each arm (except 5-2-2 was 51 patients). Most patients had lower risk disease. Hematologic improvement was achieved by 44%–56% of patients in each arm. Transfusion independency was documented in 50%–64% of patients. There was a trend for better response rates and less toxicity with the 5-day schedule of 5-azacitidine. Therefore, it is reasonable to use a shorter (5 day schedule) of 5-azacitidine in lower risk MDS.
The use of HMAs in lower risk MDS could be facilitated if enteral (oral) versions of these agents were available. Data on two of these agents, oral dac/ced (ASTX727) and CC-486 (oral azacitidine), has been published. Oral dac/ced is an oral agent that combines decitabine with the cytidine deaminase inhibitor cedazuridine.88 CC-486 is an oral form of azacytidine.95 CC-486 is not approved for MDS. Oral dac/ced has been studied in several phase 1 and 2 studies.96 In the registration phase 3 trial, patients were randomized to two different sequences of either intravenous decitabine or oral ASTX727. This allowed for intrapatient PK comparison. Results presented at ASH 2019 demonstrated virtually identical pharmacokinetic profile between both IV decitabine and the oral version. Further supporting these results are equivalence on induction of global DNA hypomethylation and early reports on clinical activity. An attenuated schedule of oral dac/ced is being studied in patients with lower risk MDS. A combination of oral azacytidine and cedazuridine is also being investigated. CC-486 is an oral form of azacytidine but with significant lower exposure due to limited absorption. CC-486 has been shown to improve survival in a study of postconsolidation therapy in AML.97 A randomized study of CC-486 has also been completed.98 In this study, 499 patients were screened but only 216 were randomized. Of importance, although the study was designed for patients with lower risk MDS, patients were required to have both red cell transfusion dependent anemia and significant thrombocytopenia. This is a rare subset of patients with very poor prognosis.43 Indeed, applying IPSS-R criteria,13 26%–30% of the patients enrolled on the study had high or very-high risk criteria. The primary end point of the trial was RBC TI. Results are also shown in Table 4. CC-486 was used at a dose of 300 mg orally daily for 21 days in 28 days cycles. RBC-TI at day 56 was 30.8% for CC-486 versus 11.1% with placebo (HR 3.6). This was 28% versus 5.6 at 84 days (HR 6.6). Duration of response was 11.1 months versus 5 months. HI-P was also significantly better for CC-486: 24.3% versus 14.3 (HR 4.6). Fewer patients progressed to AML with CC-486 than with placebo (7.5% vs. 16.7%). Despite the marked effect on red cell transfusion needs and platelet responses, treatment with CC-486 did not result in an improvement in overall survival (17.3 vs. 16.2 months). This is probably the result of early death with CC-486 and the fact that patients on the placebo arm went to receive therapy once treatment was unblinded and/or that potentially patients could be rescued with allogeneic stem cell transplantation.
5.3.1 Recommendation
Both 5-azacitidine and decitabine are used in the US in patients with lower risk disease that are transfusion dependent. Most patients treated with these agents have failed or were not candidates for growth factor support or lenalidomide. Although results from randomized studies will be needed to determine whether these agents can modify the natural history of lower-risk disease and be considered standard of care in the frontline setting or after growth factor support, recent data supports the use of these agents at lower doses particularly in patients with more adverse features. In the future oral HMAs may have a role in lower risk MDS.90, 95
Immune therapy: This is an area of controversy. It is accepted that a subset of patients with MDS are characterized by deregulation of both cellular and innate immunity.5, 99, 100 Based on this it will be logical that the use of immune-modulatory agents could have therapeutic benefit in MDS. The NIH group pioneered these approaches. Agents studied include antithymocyte globulin (ATG), cyclosporine, steroids. These therapies have been modeled after therapy of aplastic anemia.99 The NIH group also developed an algorithm to predict response to this classes of agents.101 This model included younger age, HLA-DR15 positivity, and shorter duration of transfusion dependency. Using this algorithm, the NIH group reported that alemtuzumab, an antibody against CD52, has significant activity in patients with MDS predicted to respond to immune suppressive therapy.102 The group at Moffitt Cancer Center has suggested that a CD4/CD8 ratio could also be used to predict response.103 Our group has not been capable to reproduce some of the data discussed above. Response rates with ATG observed at MDACC are significantly lower than those of the NIH.104 The most important predictor for response for us has been the presence of marrow hypocellularity.105 This is consistent with the results of Mufti et al in London.106
Also recently the impact of ATG based therapy on survival has been questioned. Data from a Swiss study comparing ATG versus supportive care indicated a higher response rate but no survival benefit.107 In this study, patients were randomized to a combination of horse ATG with cyclosporine versus best supportive care (BSC). Forty-five patients received ATG + CSA and 43 patients received BSC. By month 6, 13 of 45 patients on ATG + CSA had a hematologic response compared with four of 43 patients on BSC (p = .0156). Despite higher response rates no significant effect on survival or transformation was observed.
The NIH group has also reported on the clinical activity of eltrombopag in patients with aplastic anemia as salvage therapy108 and in addition to standard immunosuppressive therapy.109 This data was of importance due to the fact that responses were multilineage and not just restricted to platelets and significantly higher than those reported in historical cohorts with immunosuppressive therapy alone. A number of studies are investigating this agent in MDS.
Recommendation: This is a particular difficult group of patients. A majority of older patients cannot tolerate ATG and most patients are treated with some form of supportive care that could include cyclosporine, growth factors and steroids. The impact of it is not known either. In younger patients with severe hypoplastic MDS, alloSCT should be considered as soon as possible. For those that are not candidates, a combination with horse ATG is recommended. We cannot recommend the use of alemtuzumab at the present time until more data from other clinical trials is reported. The data on eltrombopag is of interest.
Allogeneic Stem cell transplantation: AlloSCT is usually not recommended in patients with lower risk disease even if they are young. This is based on data from Cutler et al using a Markov model.110 The anticipated early mortality with alloSCT cannot be overcome by the potential beneficial survival effect of alloSCT. This concept was confirmed by Koreth et al.111 Using another Markov model, the investigators analyzed the impact of reduced intensity transplant in older patients with MDS. Patients with lower risk disease did not benefited from this less toxic transplant approaches.111 In a study describing the outcomes of 438 patients with lower-risk MDS after failure to HMAs, the overall survival of patients who underwent alloSCT was significantly longer to that observed in patients not receiving further therapy, those receiving conventional chemotherapy or investigational agents (median survival of 39 months vs. 10 months, 28 months and 17 months, respectively).19
Recommendation: We generally do not recommend alloSCT in patients with lower risk disease at initial presentation. That said because of time required for donor identification, we refer all potential candidate patients for a transplant consult in anticipation of future needs. Patients that are candidates for alloSCT and that had been exposed to multiple therapies (growth factors, lenalidomide, azanuclesoides, etc.) should be considered for transplantation. These patients are also candidates for clinical trials. Patients with hypoplastic MDS that are young should be considered for alloSCT up front.
Supportive care measures in MDS: A number of interventions can be used in patients with MDS. These include the use of prophylactic antibiotics and iron chelation. No randomized data exists to make formal recommendation for any of these interventions. In our experience patients with isolated neutropenia and MDS not receiving therapy are not at significantly increased risk of infection to support recommendation of prophylactic antibiotics. Prophylactic antibiotics are commonly used in the context of active therapy for these patients. The role of iron chelation in MDS has been clarified recently thanks to the reports of the TELESTO trial112 discussed below. Data from thallasemias indicates that iron chelation has an important role in this setting. Iron accumulation is frequent in MDS. The consequences of this are not fully understood in MDS. A study evaluating the impact of iron chelation therapy on overall survival of transfusion-dependent lower-risk MDS patients reported longer median overall survival from the time of transfusion-dependence in patients receiving chelation (5.2 years vs. 2.1 years, p < .001).113 This survival advantage remained after matched pair analysis adjusting for age, frailty, comorbidity, and R-IPSS. There is controversial data with regard to the serum ferritin threshold which should trigger chelation.114 The NCCN guidelines recommend the use of chelation therapy in patients with ferritin levels above 2500 ng/mL.115 Iron accumulation could have a role in transformation to AML116 and in the increased risk of infectious complications known to occur on these patients.
5.3.2 Recommendations
We do not routinely recommend antibiotics in patients with isolated neutropenia and MDS that are not receiving some form of cytotoxic or immunosuppressive therapy. We use prophylactic antibiotics in patients receiving active therapy. We use iron chelation in patients with ferritin levels in excess of 2500 ng/mL but we consider all these patients for a clinical trial of iron chelation.
5.4 Thrombomimetic agents
The use of thrombopoietin agonists for patients with lower-risk MDS and thrombocytopenia has been explored in several studies. Initial results of a study with romiplostin117 questioned its use in patients with lower risk MDS, particularly due to complications related to disease transformation and marrow fibrosis. Results from an extension study evaluating the use of romiplostin monotherapy in 60 patients with Low or Int-1 IPSS and thrombocytopenia (defined as platelet count of ≤50 × 109 /L) reported 57% platelet responses with a median response duration of 33 weeks and only 2 patients with progression to AML.118 A number of studies are evaluated eltrombopag in MDS, another TPO agonist. Results from the phase 1 single-arm, randomized portion of the phase 2 superiority EQoL-MDS study119 evaluating the efficacy and safety of eltrombopag in patients with lower-risk MDS where recently published and reported significantly higher platelet responses (47% vs. 3%, p = .0017) and fewer bleeding events (14% vs. 42%, p = 0.0025) in the eltrombopag arm compared to placebo. No differences in leukemic transformation where observed (12% vs. 16%, p = .81). Although promising, the use of TPO agonists should be restricted to clinical trials until additional data is available.
Recommendation: Thrombomimetics agents should be used with caution and likely only in refractory patients with no other options.
5.5 Options for patients with relapsed or refractory lower risk MDS and investigational new agents in lower risk MDS
Treatment of patients with lower risk MDS is sequential. A common practice is to start growth factor support and then consider lenalidomide or an azanucleoside. In a recent large, multicenter, retrospective study including 1698 patients with lower-risk non-del5q MDS,120 early failure of ESAs was associated with a higher risk of AML progression. In a univariate and multivariable analysis, none of the second-line treatments, including HMAs, lenalidomide, and investigational agents, seemed to improve the OS of these patients significantly. Although there are limitations to a retrospective analysis with high heterogeneity, this may suggest consideration of earlier intervention with disease modifying therapy (DMT) may be an option at least for some lower-risk patients. A retrospective study evaluating the impact of the timing of DMT on the likelihood of achieving TI among 508 TD lower-risk MDS patients suggested early intervention (≤3 months from start of TD) with lenalidomide or HMAs may be associated with higher rates of TI.121 In a randomized phase III study comparing lenalidomide 10 mg daily for 21 days as single agent or in combination with ESAs in 131 RBC transfusion-dependent lower-risk ESA-refractory non-del5q MDS patients,122 the erythroid response rates after 4 cycles of therapy where 23.1% an 39.4%, respectively (p = .044) with a median response duration of 18.1 and 15.1 months, respectively (p = .47). Although growth factor support should be considered frontline, this emerging data suggest early initiation of disease modifying agents may have to be considered as reasonable options. However, randomized clinical trials will be required to support this therapeutic approach.
Patients that fail either lenalidomide or azanucleoside are candidates for clinical trials or alloSCT. There is no drug approved in the US for patients with lower risk MDS and HMA failure. The survival of these patients was calculated to be between 14 and 17 months.19
A number of agents are being studied for lower risk MDS. These include oral azacitidine90, 95 and oral dec/ced, antagonists of Toll-like receptor signaling such as OPN-205,5, 123 p38MAPK (ARRAY-614),124 siltuximab,125 bortezomib,126 ruxolutinib.127 None of these studies has demonstrated so far enough activity to warrant larger studies. Lower risk HMA failure MDS in patients not candidates for alloSCT is a major unmet need. Recently, a press release has announced that imetelstat, a telomerase inhibitor, was superior to placebo in transfusion dependent patients with lower risk MDS post ESA. A report of this data is expected in 2023.
Recommendation: At the present time there is no drug approved for patients with HMA failure lower risk MDS. Only active option is alloSCT. Otherwise, patients should be considered for investigational clinical trial.
5.6 Options for newly diagnosed patients with higher risk MDS
Options for patients with higher risk MDS have not evolved significantly since the last version of this paper. The azanucleosides remain the standard of care of a majority of patients.128
Azanucleosides: Decitabine was studied in an initial randomized comparing it to BSC.87 In this study the dose of decitabine was 15 mg/m2 IV infused over 3 h every 8 h for 3 days (at a dose of 135 mg/m2 per course) and repeated every 6 weeks. Although there was no clear benefit in terms of survival in this study, the use of decitabine was associated with a complete response rate of 9% and overall response rate of 17%. These results led to the approval of decitabine in the US. Based on the results of a phase I trial of decitabine performed at MDACC, a Bayesian randomized phase II trial of three different doses and schedules of decitabine was conducted.129 In this study a 5-day schedule of decitabine administered daily at a dose of 20 mg/m2 was shown to be superior to a 10-day or subcutaneous schedule. A multicenter phase II trial of decitabine (ADOPT) using the 5-day schedule confirmed the safety of this schedule although response rates were lower than those reported by the MDACC.130 In the ADOPT study the median number of courses administered was 5, the CR rate was 17% and the median survival was 19.4 months. No randomized survival study of a 5-day schedule of decitabine has been conducted in MDS. In parallel with this work, European investigators developed a randomized study of decitabine using the initial 3-day schedule. The major objective of the study was survival. Unfortunately, use of decitabine at this dose and schedule was not associated with improved survival in patients with higher risk MDS.131 Despite all this data, the final dose and schedule of decitabine is not fully understood. Blum et al. have indicated that a 10-day schedule of decitabine has significant activity in AML.132 Similar results have been reported by other groups in recent years.133
Oral decitabine/cedazuridine96, 134 was approved based on the results of the Ascertain trial (NCT03306264) for the same indications of IV decitabine. Then final report of this study is expected in 2023. This study confirmed the results of the initial phase 2 trial96 where oral dac/ven was shown to have almost identical PK and PD characteristics to IV decitabine. The current accepted dose of oral dac/dec is 35 mg/100 mg. The ORR in the phase 2 trial was 60% including a complete remission rate of 21% with a median survival of 18.3 months (unpublished). At ASH 2022, a subset analysis of the Ascertain trial in p53 mutated patients suggested that oral dac/ced was associated with significant survival both in patients with monoallelic or biallelic p53 mutations. In that analysis of 44 patients (35% of the total Ascertain population) the median OS for p53 mutated patients was 25 months including 29 months for those with monoallelic mutations and 13 with biallelic mutations. These figures appear larger than those previously reported in other HMA series.135 Reasons for these results are not understood and need to be explored further.
5-azacitidine has been studied in higher-risk MDS in two major randomized multicenter trials: CALGB 9221136 and AZA-001.86 In the CALGB 9221136 study, 191 patients with MDS were randomized between 5-azacitidine (75 mg/m2/day for 7 consecutive days every 28 days) and best supportive care (BSC). Median age was 68 years. Sixty percent of the patients in the 5-azacitidine group, compared with 5% of control arm patients, responded to treatment (p < .0001). The median time to leukemic transformation or death was 21 months in patients treated with 5-azacitidine versus 12 months in the BSC arm (p = .007). No significant difference in survival was observed. A landmark analysis suggested a survival advantage for patients initially on 5-azacitidine or who had crossed-over to 5-azacitidine within 6 months of inclusion on study (p = .03). A significant improvement in quality of life was documented in patients treated with 5-azacitidine compared to BSC.137 AZA-001 was a randomized study designed to test the concept that treatment with 5-azacitidine resulted in improved survival compared to a menu of standard of care options.86 These included BSC, low dose cytarabine (ara-C) or AML-like therapy. In AZA-001, 358 patients with higher-risk MDS were randomized to either 5-azacitidine (as per CALGB9221 schedule) or to standard of care. Median age of patients was 69 years. Median survival was significantly better in patients treated with 5-azacitidine versus standard of care options: 24.5 months versus 15 months (p = .0001). Progression to AML was significantly delayed, and RBC transfusion requirements and rate of infections were also significantly improved with 5-azacitidine. The survival advantage with 5-azacitidine was irrespective of age (including patients older than 75 years), percent of marrow blasts (including patients with 20%–30% blasts, now classified as AML using WHO criteria) or karyotype. This effect was significant when compared to BSC and low dose ara-C. The number of patients treated with AML-like therapy was too small to allow comparison with 5-azacitidine. No consistent biomarkers of response to azanucleosides have been developed.
5.6.1 Recommendation
The azanucleosides are the standard of care for most patients with higher risk disease. No study has compared 5-azacitidine versus decitabine. Although response rates appear to be similar, only 5-azacitidine has been associated with improvement of survival in a randomized trial. Based on this, we consider 5-azacitidine standard therapy for front line treatment in higher risk MDS.
AML-like chemotherapy: AML-like protocols in higher risk MDS have generally used classical anthracycline-araC combinations similar to those used in de novo AML.138, 139 When used in MDS or AML post-MDS, AML-like therapy results in lower CR rates (40%–60%), shorter CR duration (median duration of 10–12 months) and tend to be associated with more prolonged periods of aplasia than observed in AML. In addition, the feasibility of AML-like therapy is also reduced by the advanced median age of patients with MDS. The most important prognostic factor of response to AML-like therapy is karyotype and presence of TP53 mutations: patients with unfavorable karyotype (−7/del 7q or complex karyotype) or TP53 mutations have a low CR rate and short duration of response.140 This is of importance as, at least in the AZA-001 study,86 patients with alterations of chromosome 7 had a significant benefit with 5-azacitidine versus other therapies. Similarly, other prospective and retrospective studies report either similar or higher responses to azacitidine or decitabine in patients with MDS or AML with TP53 mutations compared to those with wild type TP53 suggesting this should be considered the standard over chemotherapy for patients with these genomic abnormalities.53, 133, 141, 142 Currently, AML-like therapy is only recommended for relatively younger patients with favorable karyotype that are candidates for alloSCT.
Recommendation: The randomized AZA-001 study was not powered to demonstrate the superiority of 5-azacitidine versus AML-like therapy. The reason for this being that most investigators did not consider their patients candidates for such therapy. Therefore, the question is who may be a candidate for AML therapy. In our practice this is restricted to younger patients with a high likelihood of response to the therapy, such as diploid patients or candidates for alloSCT. We rarely use AML therapy in older patients or in those with poor risk cytogenetics or TP53 mutation.
AlloSCT: AlloSCT is reported to be the only curative treatment of higher-risk MDS. Results from selected studies report prolonged DFS in about 30%–50% of the patients.143 However, its use is mainly restricted to younger patients with an appropriate donor. Different transplant modalities of different intensities and donor sources are now in use. Most of them remain investigational, with available studies including small numbers of patients with MDS and highly heterogeneous populations,144, 145 and therefore in our opinion all patients should be transplanted in the setting of a clinical trial. Current advances in transplant technology are allowing the consideration of older patients and alternative donors. Studies have suggested reduced intensity conditioning may be able to achieve similar relapse-free and overall survival in patients with MDS,146 and that comorbidity and disease risk indexes may be more relevant in outcomes than chronological age itself.147 This should result in greater number of older patients benefitting from this potentially curative treatment modality. A recent report studying biological assignment in patients with higher risk MDS ages 50–75 years suggested that survival on an intent to treat analysis was superior (48% at 3 years) on the donor arm versus 26% for the nondonor arm.148 Recent guidelines for alloSCT in MDS have been proposed.149
There are several relevant practical questions regarding alloSCT in MDS. These include timing of transplant; and what to do with patients that achieved a complete response to HMA prior to alloSCT. A study from the IBMTR indicated that early transplantation in higher-risk MDS was associated with longer life expectancy.110, 111 Although data suggests longer survival with SCT in patients with higher risk disease, it should be noted that curves cross close to 3 years after initiation of therapy when compared with HMAs. It will be important to identify who are long-term survivors that benefit the most from transplant. A prospective study is comparing azanucleosides use versus transplant in MDS. In terms of what to do in responding patients, no recommendation can be given at this time. For patients who experience failure to HMAs, transplant has been considered an option but a recent retrospective study seemed to indicate these patients are at high risk of post-transplant relapse compared to responding patients.150 Prospective studies will be required to clarify this. Other questions include whether or not alloSCT should be preceded by a cytoreductive regimen (with chemotherapy or perhaps HMAs). Many authors consider that when marrow blasts >10% at the time of transplant, because of the very high relapse risk post transplant, pretransplant therapy is required. A report from the EBMTR has indicated that long-term survival of patients with monosomy 7 is very poor with alloSCT.151 Similarly, as we previously detailed, two major studies152, 153 concluded that mutations in TP53, RUNX1, ASXL1, JAK2, and RAS pathway genes are associated with significantly shorter overall survival or relapse-free survival154 after alloSCT with TP53 mutations being particularly adverse. These results have significant implications for the use of alloSCT in MDS, as this suggests that the current practice of reserving transplant for poor prognostic features may not be indicated. In patients at higher risk for relapsed post alloSCT, data from a single arm trial indicated that post-transplant hypomethylating use can improve outcome.155
Recommendations: All patients potential candidates for alloSCT should be counseled regarding the possibilities of undergoing alloSCT. Optimally patients will be enrolled in an MDS specific clinical trial of alloSCT. Although alloSCT should be considered for patients with higher-risk MDS this may not be the case in patients with high risk mutations such as TP53, in whom, in our opinion, transplant may have to be considered only if an optimal donor is available, optimal pretransplant response has been obtained and if lower doses of post-transplant 5-azacitidine or decitabine are considered. Lower doses of 5-azacitidine can also be considered in patients at high risk for relapse post transplantation. I endorse recently proposed US guidelines.149
5.7 Investigational approaches for patients with higher MDS
The results of the AZA-001 trial still represent the best standard of care for patients with higher risk disease.86 In that study, patients with higher risk MDS were randomized to either azacitidine or investigator choice of therapy. Overall survival (24 months) was significantly superior to that of control. Although it is disputed that the median survival of patients with higher risk MDS is indeed 24 months and likely shorter, these results still represent the best data from a randomized trial in this setting and therefore single agent azacitidine constitutes the bar to improve. Multiple studies have attempted this. The status of combination phase III studies has recently being reviewed in detail.156
Several combinations have been studied. These are summarized in Table 5. One consists on the combination of azacitidine with the BCL-2 inhibitor venetoclax. This combination follows successful data with this combination in AML.157 Data from a multicenter trial of azacitidine and venetoclax was presented at ASH 2019 (Wei et al ASH 2019 abstract #568). This was a phase 1b study in previously untreated patients with higher risk MDS. Data from 57 patients was presented. Venetoclax was used at doses of 100 mg orally to 800 mg daily from 14 to 28 days in combination with standard dose azacitidine. The ORR was 77% with 37% of patients achieved CR. Although follow up was short, median survival had not been reached. Main issue was drug related cytopenia particularly at the higher doses of venetoclax. A single center phase 1/2 study of the combination of azacitidine and venetoclax was published in 2022.158 In this study of 23 patients, the ORR was 87% and the median survival in the previously untreated group was not reached.158 A combination of oral dac/ced is ongoing. A phase 3 trial of azacitidine +/− venetoclax (the Verona trial NCT04401748) has been completed. The second combination of significant interest is the combination of azacitidine with an antibody against CD47 known as magrolimab.159 In recently published study160 of 95 patients the combination resulted in an ORR of 75% and a CR of 33 and the median survival was not reached. A randomized registration trial known as the Enhance trial has also been completed (NCT0441748). Another potential target in MDS is TIM-3.161 This is being explored in several clinical trials with antibody known as sabatolimab (MBG453). Tamibarotene, and ATRA-like compound, is also being studied in a specific subset of patients.162
| Agent | Aza + pevonedistat180 | Aza + venetoclax158 | Aza + magrolimab160 | Aza + sabatolimab |
|---|---|---|---|---|
| Phase | 3 | 1/2 | 2 | 2 |
| Year | 2021 | 2022 | 2023 | 2021 |
| Objective | EFS | Safety, ORR | ORR | ORR |
| N | 227 | 17 | 95 | 101 |
| Population | CMML, HR-MDS, AML | HR-MDS | MDS | HR-MDS |
| ORR | 32 | 82 | 75 | 56.9 |
| CR | 31 | 18 | 33 | 19.6 |
| OS | 16.8 | Not reached | Not reached |
- Abbreviations: AZA, azacitidine; CMML, chronic myelomonocytic leukemia; CR, complete remission;N, number; NA, not available; ORR, overall response; OS, overall survival.
Recommendation: All patients with MDS should be considered for an investigational clinical trial when possible.
5.8 HMA failure still a major unmet need in MDS
The prognosis of patients with HMA failure is very poor. Natural history differs between patients still in a lower risk category where the survival is 15–17 months versus those with higher risk failure that have a survival of 4–6 months. A study with the multikinase inhibitor rigosertib in higher risk HMA failure identified two subsets of patients in the higher risk category: those with primary failure (never responded to HMA therapy) and those with secondary failure (relapsed).163 At the present time, there is no therapy that has been shown to have significant activity for this group of patients. For patients with higher risk HMA failure options that have been investigated included rigosertib,163 venetoclax,158, 164 guadecitabine among several.
Cytogenetic and molecular NGS data have value when deciding therapy, particularly AML-like approaches, in patients with HMA failure MDS. Data from a phase II trial of clofarabine and low dose ara-C in patients with relapsed refractory MDS indicated that lower doses of this combination in patients with diploid cytogenetics were associated with significant response rates and that response in this trial was associated with a median survival of 22 months.165 Therefore, selecting patients for this type of therapy (i.e., diploid cytogenetics) could be associated with improved outcomes in this group of patients. This observation is being followed with studies using lower doses of cladribine and low dose ara-C and in a phase 1 trial of CPX-351.
Recommendations: All patients with higher risk disease that have relapsed or refractory disease should be considered for an investigational clinical trial and potentially alloSCT.
5.9 Incorporating precision medicine in MDS
One of the major advances in research in MDS has been the incorporation of NGS assays, first in the laboratory, and now in the clinic. This data is not only allowing better understanding and prognostication of the disease but also design of targeted approaches for patients with MDS. Genes of interest include SF3B1, IDH2, IDH1, Flt-3, p53, and the small subset of patients with NPM1 mutated disease. SF3B1 is involved in gene splicing and is the most commonly mutated gene in MDS.166 Luspatercept is indicated for a patient population enriched for SF3B1. A selective inhibitor of SF3B1 is being studied in clinical trials.167 Agents that target IRAK4 also seem to be dependent on splicing patterns.168 IDH1 and 2 are mutated in MDS in 5%–15% of patients respectively.169 Initial data in AML studies that included a small group of patients with MDS suggested significant activity of IDH inhibitors also in MDS.170, 171 A follow up study with enasidenib has demonstrated significant activity of this compound in MDS.172 Despite that Flt-3 mutations are very rare in patients with MDS,173 they have been shown to occur in 15%–30% of patients with HMA failure.174 Those patients tend to have laboratory evidence of leukocytosis. Data from an add back design with sorafenib by Ravandi et al indicated significant activity of the addition of the Flt-3 inhibitor to azacitidine in patients with HMA failure.175 This is being followed by several small studies using second and third generation Flt-3 inhibitors in MDS. Mutation on the p53 gene have been reported in close to 10% of patients with MDS.135 Most of them are therapy related and have complex cytogenetics and therefore have a very poor prognosis. These patients tend to be resistant to conventional chemotherapy and although seem to be sensitive to HMA-based therapy, responses are short and prognosis still dismal.50 Of importance, presence of p53 mutations is associated with a very high rate of relapse post transplantation.152 Despite initial data with APR-246, p53 mutations are still a major need. Finally, around 1% of patients with MDS carry a mutation on the NPM1 gene.176 Although it can be argued that these patients constitute a rare subset of patients with AML, as those the presence of core binding factor abnormalities, the reality is that morphologically these patients are undistinguishable from other patients with higher risk MDS. A report from a small series of patients from MD Anderson indicates a very high rate of response and potentially cure with a cytarabine type of approach followed by alloSCT.176, 172
Recommendation: We advocate the use of an NGS panel at baseline and each time a therapeutic decision is going to be made in patients with MDS.
5.10 The case for total therapy incorporating alloSCT in MDS
Therapy for MDS has been dichotomized between patient candidates for alloSCT and those not candidates. Indeed, even today there is a debate of whether we need bridge therapy prior to SCT.177 This probably likely related to the fact that single agent HMA may not be very effective in achieving deep responses in MDS and not due to the fact that complete cytogenetic or molecular responses would not be associated with better outcomes in the transplant setting. This is further complicated by the fact that we observe very high relapse rates post-transplant in specific subsets of patients such as those with p53 mutations or complex cytogenetics.152 The advent of powerful new combinations with magrolimab or venetoclax argue for total therapy that could render patients MRD negative pre transplant followed with post-transplant maintenance therapy either with targeted approaches of HMAs. Further studies are needed in this area.
6 CONCLUSIONS
Better understanding of the pathobiology of MDS is resulting in newer approaches for patients with MDS. As a consequence, the treatment landscape for patients with MDS is starting to change. Luspatercept was the first agent approved for MDS since 2006. This was followed in 2020 by the oral HMA oral decitabine/cedazuridine (ASTX727). In 2023, we expect the results of the upfront trial of luspatercept in lower risk MDS (COMMANDS, NCT03682536) and of imetelstat in second line (iMERGE NCT02598661). In addition, 2 large phase 3 trials for higher risk disease have been completed (Verona and Enhance). Results with alloSCT continue to improve. All these efforts should result in the improvement of survival of patients with MDS.
ACKNOWLEDGMENTS
This work was supported in part by the University of Texas MD Anderson Cancer Center Support Grant CA016672 and by generous philanthropic contributions to the MD Anderson's MDS/AML Moon Shot Program.
CONFLICT OF INTEREST STATEMENT
GG-M reports no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
This is a review manuscript. Data reported here is publicly available.




