Transdifferentiation of B-lymphoblastic leukemia to histiocytic sarcoma after immunotherapy
Anti-CD19 chimeric antigen receptors (CAR) T cells are used to treat relapsed/refractory B-lymphoblastic leukemia (B-ALL). Tumor escape can occur by target antigen loss,1-3 and myeloid lineage switch in KMT2A4 or ZNF384-rearranged B-ALL.5 Here, we report a case of B-ALL that transdifferentiated to histiocytic sarcoma after immunomodulatory therapy.
An 8-year-old female presented with normal karyotype B-ALL that showed homozygous loss of CDKN2A/B, loss of one copy of ETV6, and somatic trisomy 21 by FISH studies. Patient was treated with Children's Oncology Group (COG) AALL0932 protocol, with an end of induction bone marrow biopsy that was positive for measurable residual disease (MRD) (0.2%). She relapsed with marrow and CNS involvement 2 years later during maintenance therapy. Hyperploidy without any structural abnormalities was noted in a subset (3 of 20 metaphases). She was treated per AALL1331 protocol followed by a matched sibling bone marrow transplant. Three months later, she again presented with blasts in the CSF and bone marrow. A next generation sequencing (NGS)-based mutation panel showed an NRAS G12S mutation. She was treated with three doses of CD19-targeting CAR T cell therapy Kymriah/Tisagenlecleucel followed by a second unrelated bone marrow transplant. She was persistently MRD positive over the next 2 years. She developed lesions in the pancreas, stomach, liver, and kidneys that were consistent with CD19+ B-ALL (Figure 1A). She was infused with anti-CD19 CAR T cells and in addition received a PD-1 inhibitor 2 weeks later given her extramedullary disease.
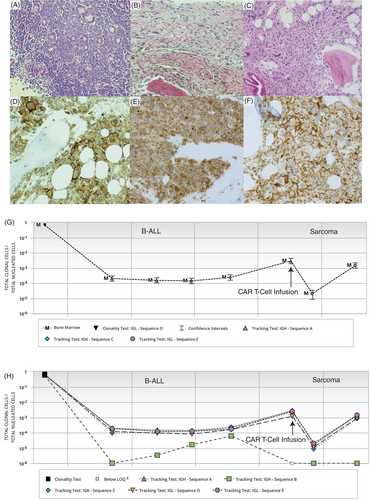
One month after the CAR T-cell infusion, a bone marrow biopsy and flow cytometry were negative for B-ALL but there was a minute focus (<1%) of atypical CD68-positive cells within the periosteum (Figure 1B). A subsequent bone marrow biopsy (Figure 1C) showed increasing involvement (20%) by an atypical infiltrate of variably sized hyperchromatic cells with irregular nuclear membranes, prominent nucleoli, and ample eosinophilic cytoplasm. Immunohistochemical stains revealed that the infiltrate was positive for CD43 (Figure 1D), CD45(dim) and CD68 (Figure 1E), with a subset of cells expressing CD4 (Figure 1F) and CD163, while negative for CD1a, CD3, CD15, CD21, CD23, CD30, CD34, CD42B, CD56, CD117, CD123, CD19, CD79a, PAX5, TdT, MPO, cytokeratin AE1/AE3, S100, ALK, GATA1, IBA1, and desmin. The overall morphologic and immunophenotypic findings were of involvement by a histiocytic sarcoma (HS). No residual/relapsed B-ALL was found by immunohistochemistry or MRD flow cytometric studies at this time point or on follow up. However, Adaptive Biotechnologies Clonoseq NGS MRD detected the same IgH, IgK, and IgL sequences as B-ALL (Figure 1G). More importantly the number of sequences detected increased (0.001%–0.01%) along with increase in sarcomatous involvement (last two time points in Figure 1G). Interestingly, one tracking IgH sequence was lost in the sarcoma samples (Figure 1H) that may be a consequence of transdifferentiation. An NGS based mutation panel (FoundationOne Heme) showed NRAS G12S mutation which had previously been documented in the patient's B-ALL. New mutations in FLCN splice site 1177-1G>A and POT1 S320 were noted in the HS. Fusions were not detected. The molecular results confirm transdifferentiation of B-ALL clone to a HS. The patient was lost to follow up and deceased.
Transdifferentiation as a means of tumoral therapy escape can rarely occur in mature B-cell lymphoma after CAR-T cell therapy6, 7 but has never reported in B-ALL. Rare cases of B-ALL with transdifferentiation to a clonally related histiocytic sarcoma have also been reported following treatment with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone therapy.8 It is difficult to be definitive as to the therapy that induced lineage switch in this neoplasm treated with multiple rounds of chemotherapy and immunotherapy. However, there might be role for immunomodulatory therapy in precipitating the switch given the temporal relationship to occurrence of HS. The role of NRAS mutation is intriguing as MAPK and RAS pathway mutations are frequently associated with histiocytic neoplasms.8 It is possible that the background of NRAS played a role in switch to HS. Interestingly PIK3, MAPK, and KRAS pathways were also implicated in loss of B cell programs after CAR T cell therapy of mature B cell lymphoma.7 FLCN and POT1 were additional mutations that were only detected in HS and not B-ALL. They are typically noted in nonhematopoietic neoplasms and could have played a role in transdifferentiation as well. In conclusion, transdifferentiation to a sarcoma is a novel mechanism by which B-ALL can escape immunotherapy pressure.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data generated or analyzed during this study are included in this published article (and its supplementary information files). Any other data will be provided on reasonable request to the corresponding author.




