The international consensus classification of eosinophilic disorders and systemic mastocytosis
Abstract
Based on new data and increased understanding of disease molecular genetics, the international consensus classification (ICC) has made several changes in the diagnosis and classification of eosinophilic disorders and systemic mastocytosis. Myeloid/lymphoid neoplasms with eosinophilia (M/LN-eo) and gene rearrangements have been renamed as M/LN-eo with tyrosine kinase gene fusions (M/LN-eo-TK). The category has been expanded to include ETV6::ABL1 and FLT3 fusions, and to accept PCM1::JAK2 and its genetic variants as formal members. The overlaps and differences between M/LN-eo-TK and BCR::ABL1-like B-lymphoblastic leukemia (ALL)/de novo T-ALL sharing the same genetic lesions are addressed. Besides genetics, ICC for the first time has introduced bone marrow morphologic criteria in distinguishing idiopathic hypereosinophilia/hypereosinophilic syndrome from chronic eosinophilic leukemia, not otherwise specified. The major diagnostic criteria for systemic mastocytosis (SM) in the ICC remain largely based on morphology, but several minor modifications/refinements have been made in criteria related to diagnosis, subclassification, and assessment of disease burden (B- and C-findings). This review is to focus on the ICC updates related to these disease entities, illustrated through changes related to morphology, molecular genetics, clinical features, prognosis, and treatment. Two practical algorithms are provided in navigating through the diagnosis and classification systems of hypereosinophilia and SM.
1 EOSINOPHILIC DISORDERS
Eosinophilia is defined as a PB eosinophil count of ≥0.5 × 109/L and hypereosinophilia (HE) ≥1.5 × 109/L. Tissue eosinophilia is defined by increased eosinophils beyond the normal range for the particular sites.1 Hypereosinophilic syndrome (HES) is defined as PB hypereosinophilia in association with tissue/organ damage.1 The causes of eosinophilia are broad and can be reactive (majority of cases), clonal proliferation due to a hematopoietic neoplasm, or idiopathic.2, 3 In eosinophilia associated with a hematopoietic neoplasm, eosinophils often bear the same molecular genetic aberrations as their progenitors and/or other clonal myeloid components.4 These hematopoietic neoplasms can be further categorized into three groups: (1) myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions (M/LN-eo-TK)5; (2) eosinophilia associated with other myeloid neoplasms, such as chronic myeloid leukemia (CML) or acute myeloid leukemia (AML) with inv(16); and (3) chronic eosinophilic leukemia (CEL), not otherwise specified (NOS).5 This first part of the current review highlights the updates in the international consensus classification (ICC)6 on eosinophilic disorders, mainly in the category of M/LN-eo-TK and further refinement of the definitions for idiopathic HE/HES and CEL, NOS.
1.1 Myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions
The disease category was created in the 2008 (4th edition) WHO classification with the original name of “myeloid/lymphoid neoplasm with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1”, to include the cases characterized by constitutive tyrosine kinase signaling as a result of the gene fusion, frequent association with eosinophilia, and except for FGFR1, often excellent responses to the first generation tyrosine kinase inhibitors (TKI). These neoplasms originate from mutated pluripotent bone marrow (BM) stem cells and may differentiate along myeloid and/or lymphoid lines, often leading to complex and heterogeneous clinical manifestations.
The category name has been changed to M/LN-eo-TK in the ICC6, 7 to emphasize the molecular genetic changes underlying these hematopoietic neoplasms that lead to constitutive TK signaling, which may be amenable to targeted therapy.3, 8 M/LN-eo-TK applies to hematopoietic neoplasms bearing the respective genetic aberration at initial presentation, not cases with such abnormalities acquired in the course of a previously diagnosed hematopoietic neoplasm. Additional important changes made in this disease category are the inclusion of M/LN with t(9;12)(q34;p13)/ETV6::ABL1 and FLT3-rearrangements as new members; and promoting PCM1::JAK2 and its genetic variants such as ETV6::JAK2 and BCR::JAK2 to formal entities from their provisional state in the revised 4th edition WHO classification. Other potential candidates for M/LN-eo-TK include rearrangements involving LYN,9 FGFR2,10 and KIT. At this point, these cases are limited to individual case studies, and more data are needed to understand their clinicopathological features and response to TKI therapy.
The updates also provide a clear guidance on how to distinguish M/LN-eo-TK presenting as B- or T- lymphoblastic leukemia (ALL) from BCR::ABL1-like B-ALL or de novo T-ALL involving one of the respective TK genes; as well as M/LN-eo-TK presenting with a mast cell (MC) proliferation from systemic mastocytosis (SM). For example, the most common genetic variant of PDGFRB, t(5;12)(q32;p13.2)/ETV6::PDGFRB, often manifests with multilineage involvement, characteristic of M/LN-eo-TK; however, some PDGFRB rearrangements, especially those with alternative partners such as EBF1, SSBP2,11 TNIP1, ZEB2, and ATF7IP, often present as de novo B-ALL without involvement of the myeloid lineage. The latter cases are best classified as BCR::ABL1-like B-ALL.12 Similarly, JAK2 fusions with certain partner genes, such as t(5;9)(q14.1; p24.1)/STRN3::JAK2, and PAX5::JAK2 are usually seen in BCR::ABL1-like B-ALL, which are, by definition, not M/LN-eo-TK. To complicate the issue, some fusion partners can be observed in either entity. For example, ETV6::JAK2 is recognized as a genetic variant of PCM1::JAK2 in M/LN-eo-TK; however, more than half of the reported cases of ETV6::JAK2 are de novo B-ALL or de novo T-ALL.13, 14 Similarly, FLT3-rearrangement can occur in de novo B-ALL and T-ALL without a myeloid lineage involvement, and these cases should be classified as BCR::ABL1-like B-ALL or de novo T-ALL rather than M/LN-eo-TK.12, 15
These decisions are driven by the general rule that, for cases of M/LN-eo-TK that initially present as B- or T-ALL, the TK gene fusion should involve the myeloid lineage in addition to lymphoblasts. In such instances, the chronic myeloid neoplasm (CMN) in M/LN-eo-TK may manifest either prior to or concomitantly or may emerge after therapy for the ALL. This concept is reminiscent of the distinction between CML presenting in B-lymphoblastic crisis and de novo BCR::ABL1+ B-ALL. For practical purposes, FISH-studies on granulocytes for detection of respective gene fusions in a clearly myeloid population may be very helpful in distinguishing M/LN-eo-TK from ALL.
An abnormal MC proliferation can be observed in M/LN-eo-TK (see below description). However, these cases consistently lack the KIT D816V mutation and show similar responses to TKI as other M/LN-eo-TK.16 In the current ICC, such cases are classified under M/LN-eo-TK if one of the TK gene fusions is detected.6 As such, it is highly recommended to test cases of putative SM for TK gene fusions, especially in cases associated with eosinophilia and lacking a detectable KIT mutation by high sensitivity molecular methods. In rare cases, a TK gene fusion and a KIT D816V mutation may coexist, and these may represent co-occurrence of two diseases with separate clones or a subclone with an acquired activating KIT mutation. These rare cases would fall within the spectrum of SM with an associated myeloid neoplasm (SM-AMN).
1.1.1 Histopathology
The disease presentations of M/LN-eo-TK cases are highly variable, and their associated morphologic features are therefore quite heterogeneous. Most of the cases present as a CMN. Peripheral eosinophilia is present in approximately 70% of the cases, most common and pronounced in cases with PDGFRA and ETV6::ABL1 rearrangements, and more variable in others. In some cases, eosinophilia may not be prominent at initial diagnosis but emerge later in the course of disease. Eosinophil morphology can range from normal to markedly abnormal (abnormal nuclear segmentation, abnormal granulation, large size or increased early forms) that is better appreciated on PB smears. For cases presenting as a CMN, the BM, and PB can show features resembling CEL, NOS, myelodysplastic/myeloproliferative neoplasm (MDS/MPN), myeloproliferative neoplasm unclassifiable (MPN-U), MDS, idiopathic hypereosinophilic syndrome (iHES), or SM with eosinophilia.
The BM of a M/LN-eo-TK CMN is frequently hypercellular with myeloid predominance, variably increased eosinophils, and often increased fibrosis. Megakaryocytes can be significantly reduced, increased, or normal in numbers; morphologically, they may be unremarkable, large with abnormal segmentation (MPN-like), small and hypolobated (MDS-like), or a mixture of both. In general, the BM usually demonstrates abnormal features suggesting a myeloid neoplasm; however, very rare cases of PDGFRA and PDGFRB rearranged M/LN-eo-TK have been reported to have unremarkable BM morphology except for increased eosinophils.17 Atypical MCs either spindle or round, with aberrant CD25 expression, scattered and in loose aggregates, were initially described as a feature characteristic of PDGFRA rearranged cases, but have subsequently been reported in cases with other TK gene rearrangements. In some cases, the MC proliferation may form dense clusters, showing histopathological features reminiscent of SM.16, 18-21 Such cases often have accompanying eosinophilia and are often diagnosed as SM-AMN before the detection of the TK fusion.
A minor proportion of patients present with acute leukemia, and the underlying CMN only becomes evident post-chemotherapy. Extramedullary infiltrates or tumoral lesions are frequent, commonly involving epidural and/or paraspinal space, or lymph nodes. These extramedullary lesions can be T-ALL/LBL (lymphoblastic lymphoma), B-ALL/LBL, myeloid sarcoma or blasts with a mixed phenotype (MPAL), or rarely a mature T-cell lymphoma, and often with various eosinophilic infiltrates. These extramedullary lesions may present at initial diagnosis or later in the course of the disease and may exhibit discordant histopathology to the PB/BM diseases, for example, lymph node biopsy showing a T-lymphoblastic lymphoma and PB/BM manifesting as CEL, NOS.
Some characteristic histopathological features have been observed in association with certain specific fusion genes. When PCM1::JAK2 presents as a MPN-like or MDS/MPN-like disorder, the BM often exhibits large aggregates of immature erythroid precursors (so-called “erythroid microtumors”) accompanied by eosinophilia and increased fibrosis. The “erythroid microtumors” are also frequently observed in extramedullary lesions, and some cases have been diagnosed as erythroblastic sarcoma.16, 22 These characteristic features should prompt FISH studies for the presence of PCM::JAK2. In contrast, the genetic variants of PCM1::JAK2, t(9;12)(p24.1;p13.2)/ETV6::JAK2 and t(9;22)(p24.1;q11.2)/BCR::JAK216, 22-24 often do not demonstrate these characteristic histopathological features. PDGFRB-rearranged cases, especially with partner gene ETV6 or BCR, frequently present with monocytosis in addition to eosinophilia. ETV6::ABL1, the new member of this category, often shows clinicopathological features reminiscent of CML with eosinophilia; however, some cases may resemble atypical CML (aCML) or essential thrombocythemia.25
Cases with FGFR1 rearrangement typically show BM features of a MPN, with eosinophilia seen in 60%–70% cases. M/LN-eo associated with t(8;13)(p11;q12)/ZMYM2::FGFR1 frequently presents with nodal (or extranodal) T-LBL admixed with scattered or perivascular myeloid blasts, being labeled as “bilineal lymphoma”.16, 26 t(8;22)(p11.2;q11.2)/BCR::FGFR1 often presents with CML-like CMN,27 or B-ALL (B-lymphoblastic phase of M/LN-eo-TK) without significant eosinophilia,28 while approximately half of t(8;9)(p12;q33)/CEP110::FGFR1 show monocytosis and tonsil hypertrophy,29 likely a presentation of extramedullary leukemia. Similarly, the histopathological features of FLT3-rearranged cases can resemble a MPN with eosinophilia, an MDS/MPN such as CMML or aCML, juvenile myelomonocytic leukemia, MDS, or SM associated with a myeloid neoplasm. Extramedullary involvement is very frequent and includes presentations such as T-LBL, mixed phenotype leukemia, myeloid sarcoma and, rarely, B-LBL, or a mature T-cell lymphoma.
1.1.2 Cytogenetics
Rearrangement of PDGFRA is the most frequent genetic abnormality within the group of M/LN-eo-TK. The PDGFRA rearrangement is usually due to an interstitial deletion of approximately 800 kb (including CHIC2) on chromosome 4q12 that leads to the FIP1L1::PDGFRA fusion. This rearrangement is cryptic on conventional karyotyping and requires FISH or RT-PCR for identification. Seven other partner genes have been reported, including CDK5RAP2/ins(9;4)(q33;q12q25), ETV6/t(4;12)(q12;p13), FOXP1/t(3;4)(p13;q12), KIF5B/t(4;10)(q12;p11), STRN/t(2;4)(p22;q12), TNKS2/t(4;10)(q12;q23), BCR::PDGFRA/t(4;22)(q12;q11). These variants are usually not cryptic (Table 1). Importantly, some AML with t(4;12)(q12;p13) have been shown to carry a PDGFRA rearrangement by FISH but insensitive to imatinib. A sequencing-based assay30 revealed ETV6 with fusion genes such as SCFD2, CHIC2, and GSX2 other than PDGFRA at 4q12. These genes are in 4q12 genomic region that are too close to be resolved by chromosome or FISH analysis. Interestingly, t(4;12)(q12;p13) with true ETV6::PDGFRA are typically found in eosinophilic disorders, whereas patients with t(4;12)(q12;p13) involving other 4q12 partners on RNA sequencing often have no eosinophilia.
| TK gene | Most common fusion | Other partner genes/variants | Typical clinical and bone marrow (BM) manifestations | Accompanying mutations | Targeted therapy |
|---|---|---|---|---|---|
| PDGFRA | Cryptic deletion 4q12/FIP1L1::PDGFRAa | BCR; CDK5RAP2; ETV6; FOXP1; KIF5B; STRN; TNKS2 | The most common M/LN-eo-TK with a male-to-female ratio: 17:1, median age in the late 40s, eosinophilia in >95% patients. Most commonly presenting as CEL-like with extramedullary involvement. |
20%–50%, including ASXL1, BCOR, DNMT3A, RUNX1, SRSF2, TET2 | Durable and complete hematologic, cytogenetic, and molecular remissions on imatinib |
| PDGFRB | t(5;12)(q32;p13.2)/ETV6::PDGFRB | >30 partners, cryptic | Male-to-female ratio: 2:1, median age in the late 40s, PB eosinophilia in around 80% patients. Age at presentation: late 40s; common presentations are CEL-like or CMML, aCML-like neoplasm, less commonly MDS |
30%–50%, including ASXL1, BCOR, DNMT3A, NRAS, STAG2, STAT5B, TET2, ZSRS2 | Durable and complete hematologic, cytogenetic, and molecular remissions on imatinib |
| FGFR1 | t(8;13)(p11.1;q12.1)/ZMYM2::FGFR1 | 15 other partners, including BCR | Male-to-female ratio: 1.5:1; median age in the late 30s; PB eosinophilia in about 70% Commonly presenting with nodal T-ALL/LBL with MPN-like features or blast phase (myeloid, B-lymphoblastic or mixed phenotype) of MPN in the BM. |
70%–80% including RUNX1, ASXL1, CSFR3, STAG2, | Complete clinical and cytogenetic responses to pemigatinib (anti-FGFR1-3), more durable in chronic versus blast phase disease (approved by FDA in 2022 for patients after 1 prior therapy); futibatinib (anti-FGFR1-4) under clinical trial evaluation |
| JAK2 | t(8;9)(p22;p24.1)/PCM1::JAK2 | ETV6 and BCR, other rarely reported RPN1, NF-E2, RUNX1, PEX14 | Male-to-female ratio: 5.5:1, median age in the late 40s; PB eosinophilia: about 70%–80% Commonly presenting as MPN or MDN/MPN-like BM with eosinophilia. Rarely in blast phase (B- and T-ALL/LBL, myeloid) of MPN |
14%–50% including ASXL1, BCOR, BCORL1, CD36, EP300, ETV6, RUNX1, SRSF2, TET2, TP53 | Hematologic and cytogenetic responses to ruxolitinib, but tend not to be durable |
| FLT3 | t(12;13)(p13.2;q12.2)/ ETV6::FLT3 |
BCR; CCDC88C; GOLGB1; MYO18A; SPTBN1; TRIP11; ZMYM2 | Male-to-female ratio: 2.2:1, median age in the mid 40s. PB eosinophilia: about 70%–80% Commonly presenting with T-ALL/LBL or myeloid sarcoma with CEL-, MPN- or MDS/MPN-like BM features |
Around 50%, including ASXL1, RUNX1, STAT5B, SRSF2, TET2, TP53, U2AF1 | Hematologic and cytogenetic responses to FLT3 inhibitors (e.g., sorafenib, sunitinib, midostaurin, gilteritinib) |
| ETV6::ABL1 | t(9;12)(q34.1;p13.2)/ETV6::ABL1 | Unknown | Male-to-female ratio: 3:1, median age in the late 40s. PB eosinophilia: about 90%–100% Commonly presenting as CML-like with eosinophilia in chronic or blast phase |
Around 40%–50% including ARID2, CDKN1B, TP53, SMC1A | More durable responses to second generation ABL1-targeting TKI (e.g., dasatinib, nilotinib) |
- Note: Modified from Tzankov et al.7
- Abbreviations: aCML, atypical CML; ALL/LBL, acute lymphoblastic leukemia/lymphoblastic lymphoma; AML, acute myeloid leukemia; CEL, chronic eosinophilic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; TK/TKI, tyrosine kinase/inhibitors. PB eosinophilia is defined as eosinophils >6% and an absolute eosinophil count >0.5 × 109/L.
- a Cases with activating point mutations of PDGFRA have been reported and should, as an exception with the family of M/LN-eo, be considered within the spectrum of M/LN-eo.
The second most common rearrangement among M/LN-eo involves PDGFRB, located at 5q31–33. So far, more than 30 partner fusion genes have been described,3 with ETV6::PDGFRB being the most common, followed by CCDC88C-PDGFRB and the rest mostly reported in individual cases. It has generally been believed that PDGFRB-rearranged M/LN-eo should demonstrate a 5q31-33 abnormality on an adequate conventional karyotype.5 However, recent studies have shown that cryptic PDGFRB rearrangements are common,16, 31 frequently occurring in partner genes other than ETV6/t(5;12)(q33;p13), such as DIAPH1,32 BCR,33 AFAP1L1, SART3, and 3GBP1,34 likely due to small deletions, inversions or alterations within complex karyotypes. Furthermore, some of the fusions may not even be detected by PDGFRB break-apart FISH, and consequently, RNA sequencing is required for identification.
Conventional cytogenetic analysis can reliably demonstrate translocations involving 8p11 associated with M/LN-eo-TK. However, due to the small size of band 8p11, the reported bands may range from 8p11.2, 8p12 to 8p21. Importantly, among the M/LN-eo with 8p11 rearrangement, only about half of the cases harbor a FGFR1 rearrangement and truly represent M/LN-eo-TK35; therefore, FISH confirmation is essential. The t(8;13)(p11;q12)/ZMYM2::FGFR1 is the most common fusion, but there are 15 additional partners. Different partner genes may have different clinical manifestations (see details above under Section 1.1.1).
PCM1::JAK2 occurs as a result of t(8;9)(p22;p24), and conversely the presence of t(8;9)(p22;p24) is almost always associated with this fusion, as demonstrated by RT-PCR or FISH.22, 36-38 It is generally accepted that the presence of t(8;9)(p22;p24) in the right clinical context is presumptive evidence of PCM1::JAK2, but FISH, at least with a probe to JAK2, is highly recommended for confirmation. M/LN-eo with alternative partners such as t(9;12)(p24.1;p13.2)/ETV6::JAK2 and t(9;22)(p24.1;q11.2)/BCR::JAK216, 22-24 demonstrate similar clinical and genetic features, and are considered genetic variants of t(8;9)(p22;p24.1)/PCM1::JAK2. Individual cases with other partner genes have been reported39 including RPN1/t(3;9)(q21;p24); NF-E2/t(9;12)(p24;q13); RUNX1/t(9;21)(p24·2;q22.1); and PEX14/t(1;9)(p36;p24.1), with clinical presentations resembling primary myelofibrosis, MDS, or “JAK2 V617F negative polycythemia vera,” respectively. Information regarding these additional partner genes is very limited. A diagnosis of M/LN-eo-TK with JAK2 rearrangement should only be made for those presenting as a myeloid neoplasm and lacking other disease defining molecular genetic changes. JAK2 fusions with other partner genes, such as t(5;9)(q14.1; p24.1)/SSBP2::JAK2,11 and PAX5::JAK240 are usually seen in BCR-ABL1-like B-ALL, which are, per definition, not M/LN-eo-TK.
ETV6::ABL1 fusion often results from a complex rearrangement involving a translocation and inversion or an insertion of ETV6 in 9q34 or ABL1 in 12p13. The fusion is often cryptic and thus FISH analysis using ETV6 and ABL1 break-apart probes, RNA-sequencing technology (RNA seq) or RT-PCR are needed for detection. The alternative splicing generates two fusion transcripts—type A (without ETV6 exon 5) and type B (with exon 5), with type B significantly more common than type A.41 ABL1 has been reported to fuse with alternate partners42; however, most of these cases present as BCR::ABL1–like B-ALL43 or de novo T-ALL.44 Therefore, for the time being, only ETV6::ABL1 M/LN-eo is included in this category. With the increased use of RNAseq in clinical samples, ABL1 fusions with other partner genes (including cytogenetically cryptic fusions) with clinical features of M/LN-eo-TK may be discovered.
FLT3 rearrangement in hematolymphoid neoplasms is uncommon, with around 30 cases reported.20, 32, 45-57 The most common is t(12;13)(p13;q12)/ETV6::FLT3, which is typically not cryptic. Other partner genes reported are ZMYM2/13q12,32, 50 TRIP11/14q32,49 SPTBN1/2p16,54 GOLGB1/3q13,48 CCDC88C/14q32,55 MYO18A/17q12,53 and BCR/22q11. In addition, there are a few cases reported in which FLT3 appears to be rearranged with yet uncharacterized partner genes at 3q27, 5q15, 5q35, 7q36, and 13q22.20, 56 Some of the fusions are cryptic, such as ZMYM2::FLT3,32, 50 and require FISH or RNA seq for identification.
The catalog of TK partner genes involved in M/LN-Eo-TK is listed in Table 1. As shown, some partner genes such as ETV6, BCR, ZMYM2, CCDC88C, can fuse to various TK genes, and each may show some characteristic clinicopathological features as described earlier.
1.1.3 Mutations
The frequencies of somatic mutations detected by next generation sequencing (NGS) vary, depending on the specific TK fusion gene and the presenting diseases. In general, mutations appear to be less frequent in cases presenting as CMN and more frequent in cases presenting as acute leukemia. PDGFRA, PDGBRB, and PCM1::JAK2 diseases have a relatively low frequency of mutations (up to 50% of cases). Mutations are reported in 20%–50% of cases of M/LN-eo with PDGFRA16, 58 including ASXL1, BCOR, DNMT3A, ETV6, SRSF2, and RUNX1. A similar frequency is reported in M/LN-eo with PDGFRB (30%–50%), with mutations involving ASXL1, TET2, BCOR, ETV6, STAG2, and RUNX1 genes.16, 58, 59 In PCM1::JAK2 M/LN-eo, mutations are reported in 14%–50% of cases,16, 22, 58 involving ASXL1, TET2, BCOR, RUNX1, SRSF2, ETV6, TP53. Mutation data are scarce for the JAK2 genetic variants t(9;12)(p24.1;p13.2)/ETV6::JAK2 and t(9;22)(p24.1;q11.2)/BCR::JAK2.
In M/LN-eo with ETV6::ABL1, although data are also limited, mutations involving ARID2, TP53, SETD2, CDKN1B, PTPN11, and SMC1A genes have been reported in approximately 50% of cases.25, 60, 61 In M/LN-eo with FLT3 fusions, mutations of ASXL1, PTPN11, RUNX1, SETBP1, SRSF2, STAT5B, TET2, TP53, and U2AF1 genes have been reported in approximately 40%–50% of cases.20 Due to the limited data, the biological role of co-operating mutations in M/LN-eo-TK is overall unclear except for M/LN-eo with FGFR1 where mutations are detected in 70%–80% of cases with around 80% of them involving RUNX1.35, 58 RUNX1 mutations have been associated with an acute leukemic presentation or progression of M/LN-Eo with FGFR1-fusions.35
The mutation data is summarized in Table 1.
1.1.4 Clinical presentations
Patient demographics vary among different TK-gene fusions. PDGFRA-rearranged cases show a very striking male predominance (male-to-female ratio of 17:1), with the usual age at onset in the late 40s but including also pediatric patients.62 Like PDGFRA, the usual age of onset for PDGFRB is also in the late 40s, although a male to female ratio is much less unbalanced (2:1). FGFR1 rearranged cases have a male-to-female ratio of 1.5:1 and often present in the late 40–50s.63, 64 B-symptoms, lymphadenopathy, and hepatosplenomegaly are common in patients with FGFR1 M/LN-eo, especially in patients presenting with T-LBL/ALL. Patients with ETV6::ABL1 M/LN-eo have a median disease onset age in the 30–40s (range 24–79 years) with no significant gender preference. Clinically, patients often present with an elevated white blood cell count (WBC), eosinophilia (>90% cases), and some with increased (>1%) basophils.25, 60 Prior to the identification of the fusion ETV6::ABL1, cases in adults were often diagnosed as MPN with marked eosinophilia, or CEL, NOS. Of note, these cases only rarely present in blast phase. Patients with FLT3 fusions have a wide range of age including pediatric patients, but median age is in 40–50s, with slightly male (2:1) predominance. M/LN-eo-TK diseases can occur in a therapy-related setting, which should be added as a qualifier. For M/LN-eo-TK, the clinical presentations tend to be associated with presenting diseases and to certain extend, the partner genes, which have been described in detail earlier, and summarized in Table 1.
1.1.5 Treatment and prognosis
Almost all patients with a PDGFRA fusion are sensitive to low dose (100 mg/day) imatinib.65 Recent data have shown that a treatment-free remission can be maintained in a proportion of patients after imatinib discontinuation, and a rapid second remission can be achieved after restart of imatinib in relapsed patients.65 Primary or secondary resistance is unusual, but if it occurs, it is linked to the T674I or D842V mutation within the ATP-binding domain of PDGFRA,66 and associated with a poor prognosis. Such patients have been shown to be responsive to second- or third-generation TKI,67 but survival is generally poor and rapid bridging to allogeneic hematopoietic stem cell transplantation (SCT) is strongly encouraged. Avapritinib has been shown efficacy in PDGFRA D842V-mutant gastrointestinal stromal tumors,68 which may be beneficial for patients with M/LN-eo-PDGFRA with D842V mutations. Like PDGRFA-rearranged cases, M/LN-Eo with PDGFRB rearrangement demonstrate an excellent response to imatinib69 with long-term complete hematologic and molecular responses. In cases presenting in blast phase of CMN or after disease progression from an initial chronic phase, induction chemotherapy and/or allogenic SCT is typically administered in addition to imatinib. However, in a case series of 17 patients70 with PDGFRA/B M/LN-eo in blast phase or sarcoma, 15 patients achieved durable complete hematologic and molecular remissions with imatinib monotherapy.
The prognosis of FGFR1-rearranged M/LN-eo is poor for cases presenting as acute leukemia or cases with leukemic progression, and SCT is considered the best curative option.71 Clinical trials using selective, oral FGFR inhibitors, for example, pemigatinib (anti-FGFR1-3)72 and futibatinib (anti-FGFR1-4)73 are ongoing with promising activity.
For cases with PCM1::JAK2 fusion, the disease course can be highly variable.22, 74 In patients presenting in chronic phase of a myeloid neoplasm, the disease may be indolent with a 5-year survival up to 80%.14, 22, 74 On the other hand, in patients who present or progress to acute leukemia, the prognosis is dismal. Targeted therapy with JAK2 inhibitors such as ruxolitinib may offer some but limited benefit24, 75 and should be used to bridge patients to allogeneic SCT, which can lead to durable disease-free survival.76, 77
FLT3-rearranged cases tend to have an aggressive clinical course or early disease progression if untreated. FLT3 inhibitor sunitinib or sorafenib monotherapy has been reported to induce rapid hematological improvements with or without complete cytogenetic response.20, 32, 46, 50, 51, 55, 57 Of reported cases, some patients have achieved a sustained remission or lived with stable disease on FLT3 monotherapy; and some been bridged to allogeneic SCT. Loss of response may occur due to acquired FLT3 N841K mutation in the activation loop of the TK domain.46 The potential role of gilteritinib78 and midostaurin has not been fully explored in these FLT3-rearranged M/LN-eo.
Patients with ETV6::ABL1 have shown variable responses to TKI targeted therapy.24, 25, 60, 79, 80 Second-or third-generation TKI24 appear to be superior to imatinib in inducing responses. Durable hematological and molecular remissions have been observed in a significant number of patients presenting in chronic phase, but not patients in blast phase. Disease progression is reported in around 30% patients that can be AML, B-ALL, T-ALL, or myeloid sarcoma, and the prognosis is dismal despite the addition of TKI to standard chemotherapy.
1.2 Idiopathic hypereosinophilic syndromes
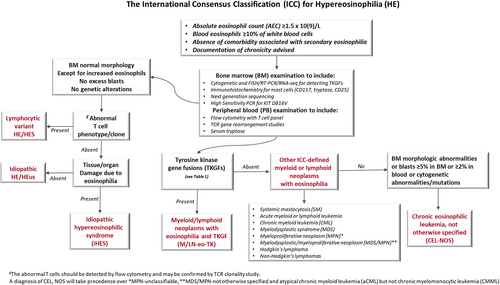
HES is defined as persistent PB HE (≥1.5 × 109/L) and/or tissue HE with associated tissue/organ damage/injury that is directly attributable to eosinophil-released cytokines or enzymes. The majority of HES are reactive due to an underlying inflammatory condition,81-83 or infection, autoimmune disorder, allergy/vaccination, or neoplasm, such as solid tumors, lymphomas, or lymphocyte variant HES (LV-HES). HES is considered as idiopathic (iHES) when an underlying primary cause (either reactive or neoplastic) cannot be identified.2, 82 HES is generally divided into1: (1) secondary HES (eosinophils are reactive and non-clonal) including lymphocyte variant-HES; (2) primary HES (associated with a hematopoietic neoplasms); (3) idiopathic HES. It is noteworthy that primary HES should be classified by the hematopoietic neoplasm according to ICC. The term of “idiopathic HE” or “HE of unknown significance” (HEus) is used to describe persistent HE (≥6 months) without associated organ/tissue damage.84, 85
It has been historically challenging to differentiate iHES/HEus from CEL, NOS (a subtype of MPN with hypereosinophilia), due to the difficulty in proving clonality in the latter. In the 2008 WHO definition, CEL, NOS was separated from iHES/HEus by the presence of increased PB or BM blasts and/or a clonal karyotypic abnormality. With the advances in NGS and its ubiquitous diagnostic application, somatic mutations associated with myeloid neoplasms can be detected in 25%–30% of patients who would have been previously classified as “iHES.”86-89 Such mutations have been found mostly in genes involved in DNA methylation and chromatin modification, such as ASXL1, TET2, EZH2, and DNMT3A, but also in other genes such as SRSF2, TP53, and SETBP1.83, 86-88 More recently, STAT5B N642H89 has been reported in some patients with a referral diagnosis of eosinophilia (1.6% of patients in one series), including patients who would be otherwise diagnosed with iHES/HEus. While the presence of a mutation provides evidence of clonality, such mutations have also been reported in aging individuals lacking evidence of a myeloid neoplasm.90, 91 Some cases with clinical features typical of iHES/HEus and with a relatively benign clinical course have also been found to carry somatic mutations.83, 92 On the other hand, some true CEL, NOS, even those with karyotypic abnormalities, may not show detectable mutations at least by myeloid neoplasm targeted NGS panels.2, 83, 92 Although the underlying cause for iHES/HEus remains to be discovered, it is acknowledged that iHES/HEus lacking convincing evidence of clonality or abnormal BM morphology of a CMN should not be considered a myeloid neoplasm and should be kept separate from CEL, NOS and other true myeloid neoplasms with HE.
1.2.1 Histopathology
Recent studies have shown that the BM of patients with iHES/HEus is usually unremarkable except for increased eosinophils17, 83, 92 (Figure 1). The cellularity is often appropriate for age, and megakaryocytes are present in adequate numbers and exhibit normal morphology. Eosinophils in PB and BM are mostly of normal forms with bilobated nuclei, though, in some cases, mild uneven cytoplasmic granulation and hypersegmentation may be observed, often in a subset of eosinophils.83, 86, 92 Mast cells (MCs) are not significantly increased. If MCs are spindly and or in clusters, further work-up to rule out SM associated with eosinophilia is warranted.
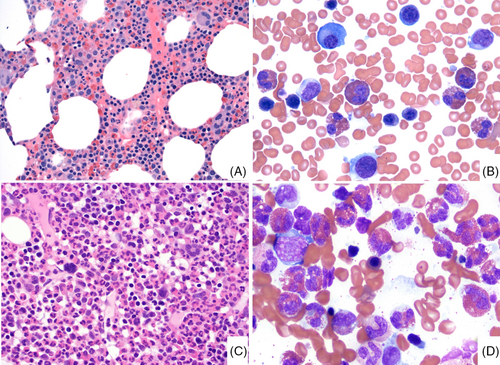
Like iHES, the BM of LV-HES is also often unremarkable except for increased eosinophilia.93, 94 However, in LV-HES, abnormal T-cells, often with a TH2 type immunophenotype (CD3-, CD4+, CD8−, CD7−/or partially lost, CD5 bright+), are identified by flow cytometry but do not form a BM infiltrate.95 Of note, T-cell clonality demonstrated by TCR PCR only is not sufficient for a diagnosis of LV-HES due to its non-specific nature. A diagnosis of iHES requires the exclusion of LV-HES. LV-HES is often sensitive to corticosteroids but carries a 5%–20% risk of progression to T-cell lymphoma with long-term follow-up. Some iHES may only present with tissue HE with a lower PB eosinophil count. Tissue HE is defined by an eosinophilic infiltrate or deposition of eosinophilic granules, significantly exceeding the normal physiologic ranges. BM HE is defined as 20% or more eosinophils of all nucleated BM cells. In some tissue biopsies, there may be only fibrosis with eosinophilic granules observed.
In contrast to iHES/HEus, the BM of CEL, NOS is often markedly hypercellular with pronounced megakaryocytic dysplasia, an increased myeloid: erythroid ratio, occasional dysgranulopoiesis, dyserythropoiesis, and myelofibrosis in 20%–30% cases, showing morphologic abnormalities similar to those seen in the BM of MDS/MPN, MDS, or MPN. In addition, eosinophils in CEL, NOS frequently demonstrate significant abnormal features (abnormal granulation, nuclear hyper- or hypo-segmentation, large size or, an increased in immature forms) (Figure 1).83, 92 A key update in the ICC is that abnormal BM histopathology is now incorporated among the required diagnostic criteria for CEL, NOS, allowing a more reliable separation from iHES/HEus.6 (Table 2).
| iHES | CEL, NOS | |
|---|---|---|
| Definition | Persistentb HE with tissue damage/dysfunction attributing to eosinophils, not caused by a known reactive or neoplastic cause | A myeloproliferative neoplasm with a prominent and persistentb proliferation of eosinophils. Blasts constitute <20% cells in peripheral blood and bone marrow, not meeting any other diagnostic criteria for AML |
| Peripheral blood | Persistent peripheral blood hypereosinophilia (eosinophil count ≥1.5 × 109/L and ≥ 10% eosinophils) | Persistent peripheral blood hypereosinophilia (eosinophil count ≥1.5 × 109/L and ≥ 10% eosinophils) |
| Organ damage and/or dysfunction | Present, attributable to tissue eosinophilic infiltrate | Not required for diagnosis, variably present in some patients |
| Etiology | No evidence of a reactive, well-defined autoimmune disease or neoplastic condition/disorder underlying HE | A myeloid neoplasm with clonal eosinophil proliferation |
| Exclusions required | Rule out neoplastic and reactive HE including lymphocyte variant hypereosinophilic syndromec | No tyrosine kinase gene fusion including BCR::ABL1, ETV6::ABL1, PDGFRA, PDGFRB, FGFR1, JAK2, FLT3 fusions not meeting any other diagnostic criteria for AML, CMML, SM |
| Bone marrow findings | Bone marrow morphologically within normal limits except for increased eosinophils | Increased cellularity with dysplastic megakaryocytes with or without dysplastic features in other lineages including eosinophils and often significant fibrosis, associated with an eosinophilic infiltrate OR There are increased blasts ≥5% in the bone marrow and/or ≥2% in the peripheral blood |
| Molecular genetics | No molecular genetic clonal abnormality, with the caveat of clonal hematopoiesis of indeterminate potential (CHIP) | Demonstration of a clonal cytogenetic abnormality and/or somatic mutation(s)d |
- a Hypereosinophilia of uncertain significance (HEus) has no tissue damage, otherwise, should follow the same diagnostic criteria for iHES.
- b Preferably a minimal duration of 6 months; however, in patients presenting with organ damage/dysfunction, immediate treatment is required, an interval of 4 weeks or two occasions with a minimum time interval of 2 weeks may suffice. Hypereosinophilia in the diagnosis of CEL, NOS is preferable 3 months, in patients required treatment, a minimum of 1 month at least two occasions.
- c The abnormal T-cell population needs to be detected immunophenotypically with or without TCR clonality by PCR.
- d In the absence of a clonal cytogenetic abnormality and/or somatic mutation(s) or increased blasts, bone marrow findings supportive of the diagnosis will suffice in the presence of persistent eosinophilia, provided other causes of eosinophilia having been excluded.
1.2.2 Molecular genetics
Theoretically, iHES/HEus should not carry cytogenetic or clonal molecular genetic abnormalities. However, it is well known that somatic mutations and rarely clonal cytogenetic abnormalities96 may be detected in individuals with no diagnostic findings of a myeloid neoplasm, such as clonal hematopoiesis of indeterminate significance and clonal cytopenia of unknown significance. Mutations have been reported in some patients otherwise having a typical clinical course of iHES, and the genes are mostly involving alterations in DNMT3A, TET2, or ASXL1 (so-called “DTA” genes). These mutations are often present as a single gene mutation and detected in a lower variant allele frequency (VAF) (<10%).83, 92
1.2.3 Clinical presentations
iHES/HEus present with blood HE (≥1.5 × 109/L) and/or tissue HE, which is persistent (6 months or more). However, for patients with iHES, to minimize further organ damage, a diagnosis of iHES may be established sooner after 4 weeks or with a repeated CBC showing persistent eosinophilia after a minimum time interval of 2 weeks.82, 97 For HEus, since there is no related tissue damage, the duration of HE is required to be ≥6 months. The median age of disease onset is in the 40–50s, showing no significant gender preference.86, 87, 92 Prior to current molecular testing, iHES was reported to have a median WBC of 20–30 × 109/L, and a median percentage of eosinophils in the range of 20%–70%. Recently, after incorporating NGS data, the Mayo Clinic study87 and the MD Anderson multicenter study92 of iHES patients both reported a significantly lower median WBC (9.4 and 11.5 × 109/L, respectively) and AEC (3.0 and 3.9 × 109/L, respectively). Cytopenia(s) are uncommon in iHES (10–20% of patients) and if present, they are usually mild. Eosinophilia associated tissue/organ damage frequently82, 84, 98, 99 involves skin, gastrointestinal organs, pulmonary/upper respiratory tract, cardiovascular system, and less frequently muscles or joints, CNS, peripheral nerves, or endocrine organs. Some patients may experience nonspecific constitutional symptoms such as recurrent fever, malaise, fatigue, or myalgia. Demonstrating a causative relation between organ/tissue damage due to eosinophilic infiltration versus a nonspecific toxic effect may be challenging in some patients due to the lack of overt abnormalities in imaging studies and/or the risks of biopsy (such as an endomyocardial biopsy, or CNS biopsy).
The clinical features of iHES/HEus are different from CEL, NOS.83, 86, 92 CEL, NOS patients are significantly older (60–70s), with a significantly higher WBC (median 25–40 × 109/L) and absolute eosinophil count (10–15 × 109/L), and much more likely to present with cytopenia(s) such as anemia and thrombocytopenia (around 50%–60% of cases), hepatosplenomegaly, and increased LDH. Of note, a significant proportion of CEL, NOS patients do not show eosinophilia-associated organ/tissue damage, which, unlike iHES, is not required for diagnosis.
1.2.4 Prognosis and treatment
The early case series of HES reported a dismal prognosis in some patients, likely due to the inclusion of true myeloid neoplasms (so-called “myeloid HES”). In recent studies where an overt hematopoietic neoplasm is excluded, iHES demonstrates a much more indolent clinical course with a long-term disease related mortality of 10%–15%.87, 92, 100, 101 Mortality is associated with age > 60 years, cardiac involvement, cytopenia (anemia or thrombocytopenia), low absolute lymphocyte count87 increased neutrophil to lymphocyte ratio,101 and hepatosplenomegaly.
For patients with iHES, treatment decisions largely depend on the pattern and extent of organ infiltration/damage. iHES with multi-organ involvement needs similar treatment considerations like ANCA-negative eosinophilic granulomatosis with polyangiitis. In general, corticosteroids are effective in producing a rapid reduction in eosinophil count or tissue/organ dysfunction in most of the patients.85, 102 However, long-term treatment with steroids to suppress eosinophilia and organ damage can carry significant side effects, with recommendations to taper once symptom control and a reduction of the eosinophil count to <1.5 × 109/L is achieved.3 Steroid resistance or intolerance occurs in about 20% of patients. Failure to control symptoms, organ damage, or persistently elevated PB eosinophil count with a prednisone dose >10 mg daily is an indication for the addition of other agents. The IL-5 antagonist mepolizumab103 has been shown to significantly reduce the occurrence of symptom flares, with marked reductions in PB eosinophilia, and can be associated with a reduced need for oral corticosteroids and other cytoreductive therapy. Benralizumab is an anti-IL-5 receptor antibody that also demonstrates activity in PDGFRA-negative HES,104 and is further being evaluated in a phase 3 randomized, double-blind, placebo-controlled study (ClinicalTrials.gov Identifier: NCT04191304).
Figure 2 is an algorithm to illustrate the ICC recommendation in the diagnosis and classification of hypereosinophilia.
2 SYSTEMIC MASTOCYTOSIS
2.1 Introduction
Mastocytosis is characterized by an accumulation of clonal MCs infiltrating one or more organs, including BM, skin, gastrointestinal tract, liver, and spleen.105 Mastocytosis can be divided into cutaneous mastocytosis (CM), systemic mastocytosis (SM), and mast cell sarcoma (MCS). CM which can present in different forms,106 is usually confined to the skin (pure CM) in children but almost always “systemic” in adults (i.e., represents a manifestation of SM).
SM is mostly a nonaggressive disease, commonly presenting as indolent SM (ISM) and more rarely as smoldering SM (SSM). Advanced SM (AdvSM) comprises aggressive SM (ASM), SM with an associated myeloid neoplasm (SM-AMN), and mast cell leukemia (MCL). MCS is a localized, destructive MC infiltration with an aggressive behavior, and around 20% of MCS are associated with underlying SM.107 If the MCS develops in a patient with SM, it is more appropriate to call those cases MCS-like progression of the SM rather than MCS. It is known that MCS associated with SM usually carries a KIT D816V mutation, whereas most cases of primary MCS lack the KIT D816V mutation.108
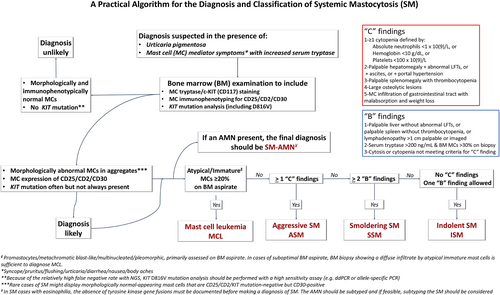
In the ICC 2022 update,6, 109 the diagnostic criteria for SM, including the major criteria and minor criteria, are mostly the same as those outlined in the revised 4th edition WHO, but some refinements have been introduced (Table 3). Some changes have also been made in the assessment of “disease burden” B findings (Figure 3). The diagnostic criteria for MCL have been modified to ensure that the entity of MCL represents a high-grade aggressive form of SM and to eliminate the term “chronic MCL”. These changes will be further discussed in the following sections.
| Major criterion |
|---|
|
| In the absence of the major criterion, at least three of the following four minor criteria must be present |
|---|
|
|
|
- a In the absence of a KIT mutation particularly in cases with eosinophilia, the presence of tyrosine kinase gene fusions associated with myeloid/lymphoid neoplasm with eosinophilia and kinase gene fusion (M/LN-Eo) must be excluded.
- b Round-cell well-differentiated morphology can occur in a small subset of cases. In these cases, the mast cells are often negative for CD25 and CD2 but positive for CD30.
- c To avoid “false negative” results, use of a high sensitivity PCR assay for detection of KIT D816V mutation is recommended. If negative, exclusion of KIT mutation variants is strongly recommended in suspected SM.
2.2 Histopathology
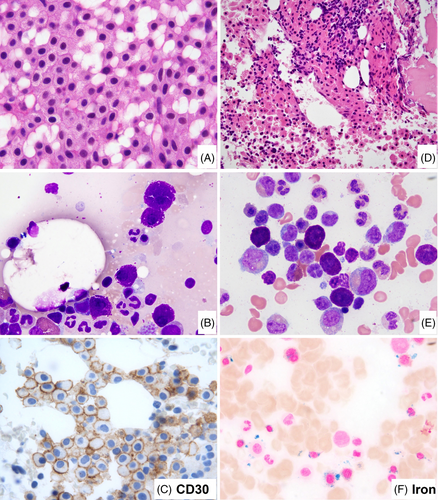
In the ICC update, there are a number of changes involving the use of histopathology in defining mastocytosis. The major criterion for the diagnosis of SM remains the presence of multifocal dense MC infiltrates (≥15 MCs in aggregates) in BM sections and/or other extracutaneous organ(s), but it now requires demonstration of CD117 (often strong) and MC tryptase (often weaker and may be partial) expression to identify and confirm the MC infiltrate.110 With the support of immunohistochemistry, the major criterion on its own can be sufficient for diagnosing SM, when M/LN-eo-TK is carefully excluded. The use of immunohistochemistry is also important to better estimate the MC infiltrate burden and to assess MC morphology. Regarding the latter, the presence of ≥25% atypical MCs in tissue sections or aspirate smears is the first minor criterion for the diagnosis of SM. Atypical MCs include spindle-shaped MCs (often also hypogranular), promastocytes, metachromatic blasts, multinucleated or highly pleomorphic MCs, and recently recognized well-differentiated MCs. Well-differentiated MCs are heavily granulated and have round central nuclei and often abundant cytoplasm111 (Figure 4). Well-differentiated MCs have been observed in various subcategories of SM, and therefore do not designate a specific subtype of SM. CD30 expression, either by flow cytometry112 or immunohistochemistry,113 can be detected in up to 80%–90% SM. CD30 is now accepted as an additional marker to CD25 and CD2 in defining immunophenotypic aberrancy of MCs,112 which is the second minor criterion in the diagnosis of SM. CD30 is particularly valuable in the assessment of SM with well-differentiated MCs that are often negative for CD25 and CD2, but frequently positive for CD30.111 Like CD25 and CD2, CD30 may be occasionally expressed in a subset of normal/reactive MCs at a low level.114 However, expression of two or more aberrant markers or strong uniform expression of at least one of the three markers is strongly associated with SM. It is important to be aware that in some cases of SM,115 the major criterion of aggregates of ≥15 MC in the BM or tissue biopsy is not present, and the diagnosis relies on the presence of three or all four minor criteria; therefore, in this setting, documentation of MC morphology and identifying a MC-associated aberrant immunophenotype become essential.
In a substantial subset of SM, there may be an associated hematological neoplasm (SM-AHN). The most common association is CMML, followed by MDS, MPN including CEL, NOS, other MDS/MPN and AML. In SM associated with hypereosinophilia, if the BM fulfills the diagnostic criteria of CEL, NOS, the diagnosis would be SM-CEL; otherwise, it will be SM with eosinophilia. A shared clonal origin has been demonstrated between SM and the associated myeloid neoplasms either by cytogenetic abnormality116 and/or KIT mutations. In rare cases, SM may present with a concurrent lymphoid neoplasm or plasma cell neoplasm, and in such cases these two components are instead considered clonally unrelated, co-incident diseases.116-118 In the ICC 2022 update, SM-AHN is now limited to the association with a myeloid neoplasm (SM-AMN). A high degree of suspicion for an underlying myeloid neoplasm should be raised by the presence of significant cytopenia(s) or cytosis (leukocytosis, monocytosis, eosinophilia, thrombocytosis), splenomegaly, elevated LDH, high KIT D816V variant allele frequency, as well as the detection of additional somatic mutations in genes associated with myeloid malignancies. Morphologic assessment of the BM should include BM cellularity, megakaryocyte morphology, dyserythropoiesis, dysgranulopoiesis, monocytosis, eosinophilia, and blasts. The AMN should be subclassified according to established criteria. On the other hand, if a KIT mutation is detected in a myeloid neoplasm, performing CD117 and MC tryptase stains is strongly recommended to identify an occult associated accumulation of neoplastic MCs,119 especially in CMML and AML with t(8;21).
In SM, disease burden and aggressiveness are measured by B- and C-findings. B findings refer to organ enlargement without organ failure, while C-findings refer to MC-related organ damage. In the ICC update, the vague morphological description in the second criterion of the B finding in the revised 4th edition of the WHO “signs of dysplasia or myeloproliferation in non–MC lineage(s), but criteria are not met for a definitive diagnosis of an associated hematological neoplasm” is now removed to avoid ambiguity in disease definition. As a result, the first part of the B finding is now simplified to “cytopenia (not meeting criteria for C findings) or -cytosis. Reactive causes are excluded, and criteria for other myeloid neoplasms are not met” (Figure 3).
Another important change is in the definition of MCL. In the past classifications including the revised 4th edition of the WHO, MCL was simply defined as SM with ≥20% MCs on BM aspirate smears, regardless of MC morphology or C findings. In most cases of SM, MCs are bland-appearing spindled cells associated with significant reticulin fibrosis, yielding very few MCs in BM aspirates even in cases with a high MC burden. The cases with ≥20% MCs on BM aspirate smears are those without significant fibrosis and mostly round-shaped MCs with high grade cytology (i.e., atypical immature MCs, see below) or with well-differentiated morphology. The latter have been described in a proportion of cases of the so-called chronic MCL.120 Due to the same reason of BM aspirate for MCs, cases of ASM with 5%–19% MCs on BM aspirates have been found to be similarly aggressive and are referred to as “ASM in transformation.”108 Of note, MCL is the only disease in the hematolymphoid neoplasm classification which exclusively relies on BM aspirate smear count independently of the biopsy. In the ICC 2022 update, MCL is redefined as a subset of SM with atypical immature morphology in 20% or more of the BM cells. Atypical immature MCs include metachromatic blasts, promastocytes, multinucleated, anaplastic, or highly pleomorphic MCs.121, 122 Metachromatic blasts123 are blast-like (immature chromatin, with or without nucleoli) with metachromatic granules; while promastocytes are immature MCs with bilobated nuclei and cytoplasms packed with metachromatic MC granules (Figure 5). Spindled MCs and MC with well-granulated morphology (well-differentiated MC), though different from normal MC, are not considered as atypical immature MCs. The ICC does not recognize the still poorly characterized entity termed chronic MCL.120 Following the ICC approach, if the aspirate is suboptimal or a dry tap, a BM biopsy showing a diffuse MC infiltration with at least 20% of atypical immature MCs by CD117, and/or tryptase immunohistochemistry stains is sufficient to support a diagnosis of MCL. In addition, the ICC has further removed “aleukemic” or “leukemic” from the subclassification of MCL. Of note, the majority of MCL are aleukemic in that ≥10% circulating MCs are very infrequent.124 Recently, by flow cytometry, circulating MCs were detected in nearly half of the patients with ISM, almost all patients with AdvSM and SSM.125
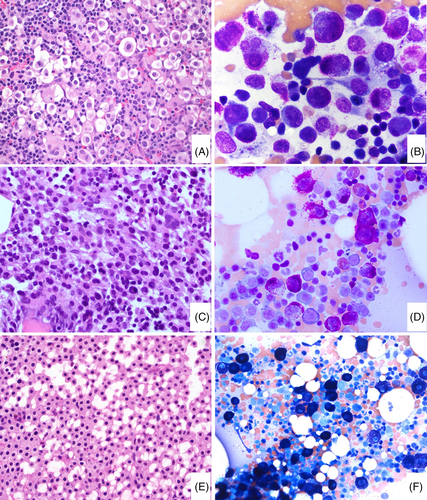
2.3 Cytogenetics
An abnormal karyotype is reported in 10%–15% SM in two large series.126-128 The majority of the clonal abnormalities are identified in patients with SM-AMN (25%–30%), followed by MCL (15%–20%) and aggressive SM (<10%). The karyotypic abnormalities, which are not unique to SM, are likely related to the underlying AMN (CMML, MDS, MDS/MPN, or MPN). These include −7, +8, or complex karyotypes, and less frequently, del(5q) or del7q. In ISM, clonal cytogenetic abnormalities are extremely rare. Whenever present, they are low risk abnormalities such as del(20)q, +Y, del(12)p, or inv(2). An abnormal karyotype is more frequently associated with male gender, older age, and cytopenia(s).127 While an abnormal karyotype did not identify it as an independent prognostic factor in patients with SM,127 a study of MCL patients found that it was associated with inferior outcomes.126 Monosomy 7 and complex karyotypes in advanced SM (including SM-AMN) have been reported to be associated with disease progression to MCL124 and secondary AML.128
2.4 Molecular genetics
KIT D816 mutation is detected in >90% of SM, mostly in the indolent forms of SM, but >50 other rare KIT mutations have also been described in SM.129 Over 75% of ISM and SSM have an isolated KIT mutation without other co-mutations.130, 131 Due to a low yield of MCs in BM aspirates, the use of a high sensitivity PCR assay for KIT D816, such as quantitative allele-specific polymerase chain reaction (PCR) or digital droplet PCR (ddPCR) assays with a sensitivity down to 0.01%–0.1%,132 is highly recommended in suspected SM whenever NGS fails to reveal a KIT mutation. While KIT D816 mutation is uncommon or absent in MCS or SM with well-differentiated morphology as well as in some AdvSM, other activating KIT mutations are not infrequently encountered.107, 111, 129, 133 As these other activating KIT mutations can render the MCs responsive to TKI, complete KIT gene sequencing should be strongly considered.134-136 If a KIT mutation is absent, particularly in cases with eosinophilia, TK gene fusions associated M/LN-Eo-TK must be excluded. As previously discussed, M/LN-eo-TK may show morphologic findings similar to those seen in true SM.
KIT D816V mutation in non-MC lineages can be found in ISM, but is much more frequent in AdvSM, particularly SM-AMN. The KIT D816V VAF in PB and BM correlates with the subtype of SM, multilineage involvement, serum tryptase level, organ involvement, disease progression and survival.117, 125, 137 In SM-AMN, especially SM-CMML and SM-MDS, a KIT D816V mutation has been clearly demonstrated not only in SM, but also in the myeloid cells.117 The measurement of KIT D816V VAF has been incorporated as a benchmark for treatment response in studies of non-advanced and advanced SM.138, 139 Additionally, a 10% or higher KIT D816V allele burden has been proposed as a novel additional B-finding108 and is included in the 5th edition WHO Classification.97 While a correlation between KIT D816V VAF and disease burden may be observed, it was felt by the ICC that more evidence is needed before a precise threshold can be determined and clinical utility assessed. In fact, a recent study140 showed that the presence of ≥3.5% (not ≥10%) KIT D816V-mutated cells as well as a dynamically unstable KIT D816V mutation burden (increase or decrease over time) in blood and/or BM are associated with a significantly shortened progression-free survival (PFS). In addition, a high mutational VAF can be due to a clonally related concomitant myeloid neoplasm, which may or may not be readily apparent at the time of diagnosis of SM, or may be unmasked when SM-directed therapy is administered.
In contrast to ISM and SSM, the genetic profile of AdvSM is more complex, with 60%–70% of cases showing mutations in addition to KIT.130, 141-145 These additional somatic mutations are strikingly similar to those encountered in various myeloid neoplasms such as SRSF2, ASXL1, RUNX1, TET2, DNMT3A, JAK2, CBL, and NRAS. The genetic profile and the VAF of individual genes may substantially affect clinical phenotype, response to treatment, and prognosis. Mutations in one or more of the SRSF2, ASXL1, and/or RUNX1 (so-called S/A/R gene panel) genes are associated with a poorer prognosis in AdvSM, including MCL.141, 143 Therefore, it is recommended to test other pathogenic mutations in genes with known prognostic impact in SM patients for further identification of patients at higher risk. It is noteworthy that all of the above molecular markers should be used in combination with other disease features for accurate risk stratification of patients with SM.
2.5 Clinical features
The clinical presentations vary among different subtypes of SM that are closely related to B- and C- findings. In the ICC 2022, the “burden of disease” B-findings are largely unchanged but simplified and refined (Figure 3). In addition to removing the description of BM morphology as discussed above, the third criterion has been modified to specify “splenomegaly” as “palpable splenomegaly without features of hypersplenism including thrombocytopenia” and “lymphadenopathy” as “lymph node size of >1 cm” (a threshold of >2 cm is used by the ECNM-AIM consensus criteria108). No changes have been made to the “C”-findings (Figure 3).
ISM is the most common form of SM,146 defined by less than 2 B-findings and absence of C-findings. ISM has a median onset age of 45–57 years.146-148 Its presenting symptoms include maculopapular skin lesions, itching, flushing, abdominal pain, acid reflux, nausea, diarrhea, brain fogginess, and musculoskeletal pain. These symptoms significantly affect the quality of life in about two thirds of patients. Secondary osteopenia and osteoporosis are reported in up to one third of patients. These may be the presenting symptom of the disease.149, 150 Isolated BM mastocytosis (BMM) is a clinicopathologic variant of ISM (ICC), which account for 25%–60% of all ISM.150, 151 BMM is characterized by absence of skin/cutaneous lesions, isolated BM involvement with a lower MC burden, tryptase level < 125 ng/mL, no B finding, older age, and male predominance.149, 150, 152 The incidence of hymenoptera allergic/anaphylaxis reactions to the sting by a bee, wasp or other hymenoptera insects due to an IgE-mediated allergy to its venom,149, 150 is significantly higher in BMM than other ISM (62% vs. 16%).150 Overall, the prognosis of ISM is excellent, with reported disease progression to AdvSM in <3% patients.148
SSM is characterized by a high MC burden, defined by the presence of ≥2 B-findings but no C findings. SSM has a higher rate (9%–15%) of progression to AdvSM (up to 7%) than ISM.146-148 In contrast, ASM has ≥1 C-finding(s) due to SM-induced organ damage. ASM can occur de novo or as progression from ISM or SSM.146, 147, 153, 154 Skin lesions of ASM are less common than ISM (present in ~50%), but constitutional symptoms (60%), hepatosplenomegaly (50%), and lymphadenopathy (30%) are frequent.155 While patients with ISM may have osteoporosis, patients with ASM often present with osteosclerosis and may present with pathologic fracture(s) due to lytic bone lesions. SM-AHM should be excluded before making a diagnosis of SSM and ASM.
In SM-AMN, the SM and the underlying myeloid neoplasm may be diagnosed at the same time (67%), or at a variable interval (3–370 months) apart.156 In patients with SM-AMN, skin lesions are present in 60%–70% of cases156 and cytopenia, cytosis, hepatosplenomegaly, increased LDH are common, depending on the type of AMN. It can be extremely challenging to attribute potential C findings, including clinical symptoms, signs of organ involvement, and abnormal laboratory findings, to SM versus the concomitant AMN. BM biopsy to assess MC burden as well as the AMN component, especially blast count, may provide important information for clinical management and therapeutic decision making, as the prognosis of SM-AMN tends to depend largely on the type and stage of the AMN.
Figure 3 provides a practical algorithm for the diagnosis and classification of SM following the ICC criteria.
2.6 Prognosis and treatment
The prognosis and survival in ISM are favorable. Progression of ISM to SSM occurs in around 2% of patients and to AdvSM in 1%–3%.147, 148, 154, 157 Progression of SSM to AdvSM occurs in up to 7% of patients.158 For patients with ISM and SSM, the treatment is to manage SM mediator symptoms and avoid triggers that may exacerbate symptoms. Antihistamine medications (anti H1 and/or H2 receptor) are recommended depending on the constellation of mediator symptoms. ISM patients (as well as patients with all mastocytosis variants) are encouraged to carry epinephrine autoinjectors to mitigate potentially life-threatening episodes of anaphylaxis. For patients who are prone to anaphylaxis due to bee/wasp venom allergies, venom immunotherapy to induce immune tolerance has shown to provide protection.159 Anti-IgE antibody treatment (e.g., omalizumab) has shown benefit in patients to prevent life-threatening reactions.160 Other treatment for ISM/SSM includes leukotriene inhibitors, cromolyn sodium, and ketotifen. Bisphosphonates and RANK ligand inhibitors (e.g., denosumab) may be considered for osteoporosis which can be more frequent and severe in patients with mastocytosis. Historically, PEG-interferon-α, corticosteroids, and cytoreduction with cladribine have been reserved for patients with severe, refractory symptomatic ISM; however, both short- and longer-term toxicities, need to be considered carefully before using such agents in these patients who otherwise exhibit a (near) normal life expectancy. Highly selective KIT D816V inhibitors (e.g., avapritinib and bezuclastinib) are currently being assessed in clinical trials of ISM patients with refractory symptoms despite best supportive care. Validated mastocytosis-specific patient-reported outcome measures should be used for treatment response assessment.161 In trials of KIT inhibitors in ISM, BM MC burden, serum tryptase level, and KIT D816V VAF are being evaluated as biomarkers of response but do not necessarily correlate with the severity of mediator symptoms and quality of life.
AdvSM carries a poor prognosis, typically with a median survival of less than 4 years.146, 147, 162 Midostaurin is a multikinase/KIT inhibitor that targets not only D816V-mutated KIT but also wild-type (WT) KIT, PDGFRα/β, VEGFR2, and FLT3.163 Avapritinib is a potent and highly selective oral type 1 multi-kinase inhibitor with activity against KIT D816V.164-167 Based on trials of AdvSM patients, midostaurin163 and avapritinib,164, 165, 167, 168 were approved by the FDA for frontline treatment of AdvSM, in 2017 and 2021, respectively.169 Bezuclastinib is an oral highly selective TKI with minimal brain penetration which is currently being evaluated in AdvSM.170 In patients with pure MCL and ASM without an AMN component, treatment with midostaurin or avapritinib generally result in better responses compared to patients with SM-AMN. However, treatment decisions for SM-AMN should be individually tailored, prioritizing the treatment for the component felt to be mostly contributing to the patient's clinical symptoms and organopathy. This should include consideration of the patient's mediator symptoms, complete blood count, BM findings including MC burden and blast count/stage of AMN, tryptase level, presence and severity of organ damage (e.g., cytopenias, liver function abnormalities, hypoalbuminemia with weight loss), and molecular profile including KIT D816V VAF and presence of other myeloid molecular abnormalities, especially the poor-risk SRSF2/ASXL1/RUNX1 mutations. The SM component often responds to KIT targeting agents, with molecular remissions of KIT D816V achievable with highly selective KIT D816V inhibitors such as avapritinib. However, progression of disease is usually related to the AMN component. In this regard, how best to combine KIT inhibitors with AMN-targeted therapy is a major focus of future clinical trial development in AdvSM. Allo-SCT may be considered for patients with SM-AMN, especially in younger patients with good performance status who have achieved a pre-SCT complete remission or significant reduction of the MC component. In the KIT inhibitor era, the ability of new potent TKIs (e.g., avapritinib) to elicit pathologic complete remissions and deep molecular responses is increasingly attainable, which should make more patients eligible for transplant if they are felt to be appropriate candidates.171 The role of KIT inhibitors in the posttransplant setting to reduce relapse also merits investigation.
CONFLICT OF INTEREST STATEMENT
Jason Gotnib: Institutional funding for trials: Blueprint Medicines, Cogent Biosciences, Incyte Consultation and/or service on Steering Committees/central review Committees: Blueprint Medicines, Cogent Biosciences, Incyte. Andreas Reiter: Institutional funding for trials: Blueprint Medicines, Cogent Biosciences, Incyte, Novartis Pharma. Consultation and/or service on Steering Committees/central review Committees: Blueprint Medicines, Cogent Biosciences, Incyte, Novartis Pharma. Alexandar Tzankov: Steering Committee: Blueprint Medicines. No conflict of interest relevant to this article for other authors.
Open Research
DATA AVAILABILITY STATEMENT
There is no original study data associated with this article.




