Impact of type 2 diabetes on mortality, cause of death, and treatment in chronic lymphocytic leukemia
Susan L. Slager and Carsten Utoft Niemann contributed equally to the work.
Abstract
Age-related comorbid conditions are exceedingly common in patients with chronic lymphocytic leukemia (CLL). As the prevalence of type 2 diabetes (T2D) is predicted to double during the next two decades, a better understanding of the interplay between CLL and T2D is of increasing importance. In this study, analyses were performed in parallel in two separate cohorts, based on Danish national registers and the Mayo Clinic CLL Resource. The primary outcomes were overall survival (OS) from time of CLL diagnosis, OS from time of treatment, and time to first treatment (TTFT), studied using Cox proportional hazard regression analysis and Fine-Gray regression analysis. In the Danish CLL cohort, the prevalence of T2D was 11%, in the Mayo CLL cohort, it was 12%. Patients with CLL and T2D had shorter OS both from time of diagnosis and from first-line treatment for were less likely to receive treatment for CLL compared with patients with CLL and without T2D. The increased mortality was largely driven by an increased risk of death due to infections, especially in the Danish cohort. The findings of this study emphasize a substantial subgroup of CLL patients with co-occurring T2D with an inferior prognosis and a possible unmet treatment need requiring additional interventions and further research.
1 INTRODUCTION
In chronic lymphocytic leukemia (CLL), the median age at diagnosis is 72 years, and most patients do not require treatment until after several years.1, 2 The median survival for patients with CLL is approximately 13 years, and it has increased in the past few years likely owing to the introduction of highly effective novel agent targeted therapies.2-4 The large proportion of older patients with CLL carries a correspondingly high prevalence of age-related comorbid conditions both at the time of CLL diagnosis (35%–52%) and at the time of initial treatment (54%–57%).5-9 We and others have previously demonstrated that comorbid conditions are associated with an increased mortality in CLL, due to causes both related and unrelated to CLL.5, 6, 10-16 Diabetes is one of the most common comorbid conditions with a prevalence of 8%–21% at the time of CLL diagnosis.6, 7, 13 During the next two decades, the prevalence of diabetes is projected to double in the general population, partly due to an increasing incidence of type 2 diabetes (T2D).17-20 While some comorbid conditions may have limited treatment options and poor potential for improvement of management, treatment for T2D has evolved significantly over the last decades. New treatment agents such as SGLT2-inhibitors and GLP1-analogs have displayed survival benefits and lower risk of diabetes-related complications in combination with first-line treatment with metformin in T2D.21-26
An association between diabetes (types 1 and 2 combined) and all-cause mortality has been reported in a population-based cohort of patients ≥71 years with CLL, although no association remained after multivariable modeling.27 We have recently demonstrated that diabetes (types 1 and 2 combined) is associated with increased mortality due to both CLL-related and CLL-unrelated deaths in the Danish CLL cohort,15 while previous studies based on the Mayo Clinic CLL cohort have only revealed increased mortality due to CLL-unrelated deaths.6, 7 Moreover, a study of explicitly T2D and non-Hodgkin lymphomas (NHL) demonstrated an association between T2D and increased risk of death related to NHL.28
We present characteristics of patients with both CLL and T2D and on associations of T2D with time to first CLL treatment, overall survival from CLL diagnosis, and overall survival from the first treatment. This study features two large cohorts: the Danish National population-based CLL cohort and the Mayo Clinic CLL cohort.
2 METHODS
2.1 Data sources and study population
The study included two separate cohorts based on data from (1) the Danish CLL Registry and the Danish Cancer Register (Danish cohort) and (2) the Mayo Clinic CLL Resource (MCCR, Mayo cohort).29-34
In Denmark, all citizens are provided access to free health care services, which are monitored in national administrative registers.35 At birth or immigration, citizens are given a unique personal identification number (CPR), which is used for all contacts with health care services.36 The CPR number enables the linkage of person-level data across national administrative registers and clinical quality registers.37 The Danish CLL Register is a national clinical quality database established in 2008 in which it is mandatory for all physicians to provide predefined patient data.29
The MCCR has enrolled individuals with CLL who were clinically seen in the Division of Hematology at Mayo Clinic Rochester since 1995. Diagnoses, including those diagnosed before 1995, were confirmed by a hematopathologist using the National Cancer Institute working group criteria and updated using the International Workshop on CLL (iwCLL) criteria. All clinical data are abstracted from medical records and are maintained on a prospective basis.32-34
Patients diagnosed with CLL or small lymphocytic lymphoma (SLL) (henceforth, referred to as CLL) from 2008 to 2017 in the Danish CLL registry or 2002–2018 in the Danish Cancer Register were included in the Danish cohort; patients in the MCCR who were diagnosed with CLL from 1993 to 2021 were included in the Mayo cohort. To reduce the risk of diagnostic misclassification of CLL, patients <30 years of age at the time of CLL diagnosis were excluded. Patients were followed in the Danish Civil Registration System or the MCCR from CLL diagnosis until the earliest of death, emigration, or end of follow-up (Danish cohort 2018, MCCR 2021). Data on causes of death were obtained through the Danish National Register of Causes of Death and the MCCR.38 Deaths caused by CLL, Richter transformation, other hematological malignancies, other malignancies, or infections were categorized as CLL-related, and all other causes were classified as non-CLL-related, consistent with previous definitions used by this group.15 Prognostic baseline CLL characteristics, such as clinical stage (Binet or Rai), cytogenetic aberrations, immunoglobulin heavy-chain variable (IGHV) region gene mutational status, and β2-microglobulin level, as well as the type of first-line treatments, were available from the Danish CLL register and the MCCR.39 In the Danish cohort, data on prognostic factors were only available for patients diagnosed after 2008 included in the CLL register.
In the Danish cohort, T2D was defined as having an International Classification of Diseases-10 discharge diagnosis of T2D (E11) in the Danish National Patient Register or at least one prescription for non-insulin antidiabetic drugs (Anatomical Therapeutic Chemical Classification code A10B) in the Danish Prescription Register.40, 41 The definition of T2D based only on an antidiabetic prescription of metformin was not sufficient for females 20–39 years of age, as metformin may be used as a treatment for polycystic ovarian syndrome.42-44 In the Danish cohort, the prevalence of T2D was assessed at CLL diagnosis and first CLL treatment. In the Mayo cohort, prevalent T2D was obtained from a self-reported questionnaire at consent. In the Danish cohort, patients with (1) a prescription for insulin prior to the age of 30 years, (2) a diagnosis of type 1 diabetes (T1D) prior to the age of 30 years (E10), or (3) a diagnosis of T1D prior to their T2D diagnosis and no prescriptions for non-insulin antidiabetic drugs were classified as having T1D and were excluded from the study.41 Information on diabetes-related complications and the number of antidiabetic drugs used were collected through the same sources as were used to identify T2D for the Danish cohort.45 For the Mayo cohort, information on overweight and obesity was obtained from the questionnaire. In the Danish cohort, a look-back period of up to 20 years was used for discharge diagnoses (up to 25 years for T1D and T2D diagnoses) and 1 year for prescriptions.
Patients with CLL in the Danish cohort were matched on sex, date of birth, and region of residence with up to 10 comparison persons from the background population without CLL at the time of diagnosis of the index patient. The presence of T2D and related characteristics were obtained for individuals in the comparison cohort using the same methods as for patients with CLL.
2.2 Statistical analysis
Analyses were performed in the two cohorts in parallel. Descriptive statistics were calculated and reported as frequencies and medians with interquartile ranges (IQR) or ranges. The primary outcomes from CLL diagnosis date in all patients were overall survival (OS) and time to first treatment (TTFT). The primary outcome from the first CLL treatment date in treated CLL patients was OS. Cause-specific mortality from CL diagnosis and event-free survival (EFS, a composite endpoint of death or second treatment) from the time of treatment was also of interest.
Univariable and multivariable Cox proportional hazards regression analyses (Cox PH) were performed to investigate the association of T2D with OS and EFS; estimates are reported as hazard ratios (HRs) and 95% confidence intervals (CI). Proportional hazard assumptions were visually assessed and were met in all variables examined. Fine-Gray regression models were fitted for the association of T2D with TTFT, treating the death as a competing risk, and with cause-specific mortality, treating death due to other causes as a competing risk. Multivariable Cox PH models were adjusted for age, sex, and calendar year. Sensitivity analyses were performed additionally adjusting for the International Prognostic Index for Chronic Lymphocytic Leukemia-International Prognostic Index (CLL-IPI) score.46, 47 Multivariable Cox PH models estimating the association of T2D with OS and EFS in all treated CLL patients were adjusted for age and sex; the treatment regimen was evaluated in additional models. Associations of type of CLL treatment with OS from the first CLL treatment in patients with both CLL and T2D were conducted using univariable Cox PH models; multivariable Cox PH models adjusted for age, sex, and IGHV mutational status. Kaplan–Meier and cumulative incidence curves visually displayed the data, while statistical inferences were primarily based on adjusted parameter estimates. Data analysis was performed using SAS software, version 9.4 (SAS Institute, Cary, NC) and R software version 3.5.2. Analysis of Danish data was performed on servers hosted by the Danish Health Data Authority.
2.3 Ethics
The study was approved by the Danish Health and Medicine Authorities (jr. no. 3-3013-1141/1), Danish National Ethics Committee (1804410), the Danish Data Protection Agency (jr. no. RH-2015-96 03856) and SSI QC and Compliance (jr. no. 21/00805). No consent was required as all data used for the Danish cohort was from existing Danish national registers. In accordance with Danish legislation, all analyses were performed on pseudonymized data, and subgroup findings of <5 individuals were not reported. The Mayo Clinic Institutional Review Board approved the study.
3 RESULTS
3.1 Baseline characteristics at CLL diagnosis
In total, 10 515 patients with CLL were included in the study: 7446 from the Danish cohort and 3069 from the Mayo cohort (Table 1). The Danish and Mayo cohorts had a median follow-up time of 4.9 and 5.4 years, respectively. In the Danish cohort, 802 patients (11%) had T2D at the time of CLL diagnosis and in the Mayo cohort 354 (12%). The median age at CLL diagnosis was 71 and 64 years, and 60% and 68% of the patients were male in the Danish and Mayo cohorts, respectively. In both cohorts, patients with T2D tended to be older and male compared to patients without T2D. The distributions of Binet/Rai stage, IGHV mutational status, and fluorescence in situ hybridization (FISH) status were similar for patients with and without T2D in both cohorts. Intermediate or high CLL-IPI scores were more prevalent in patients with T2D, primarily due to a higher prevalence of elevated β2-microglobulin (B2M) levels. Information on B-cell count was available in the Mayo cohort on 47% of patients, with a similar median B-cell count at diagnosis in patients with T2D (7.7 × 109/L, IQR: 4.8–14.5) and patients without T2D (8.8 × 109/L, IQR 5.5–17.8).
| Danish cohort | Mayo cohort | |||||
|---|---|---|---|---|---|---|
| CLL and T2D | CLL and no. T2D | Total | CLL and T2D | CLL and no. T2D | Total | |
| n (%) | n (%) | |||||
| Number of patients | 802 (11) | 6644 (89) | 7446 (100) | 354 (12) | 2715 (88) | 3069 (100) |
| Sex | ||||||
| Female | 274 (34) | 2719 (41) | 2993 (40) | 87 (25) | 890 (33) | 977 (32) |
| Male | 528 (66) | 3925 (59) | 4453 (60) | 267 (75) | 1825 (67) | 2092 (68) |
| Age at CLL diagnosis | ||||||
| Median (IQR/range) (Danish/Mayo) | 72 (67–80) | 71 (63–78) | 71 (63–78) | 68 (35–89) | 63 (21–97) | 64 (21–97) |
| <65 | 151 (19) | 2074 (31) | 2225 (30) | 131 (37) | 1469 (58) | 1600 (52) |
| 65–71 | 235 (29) | 1561 (23) | 1796 (24) | 98 (28) | 610 (22) | 708 (23) |
| 72–79 | 227 (28) | 1648 (25) | 1875 (25) | 95 (27) | 413 (15) | 508 (17) |
| ≥80 | 189 (24) | 1361 (20) | 1550 (21) | 30 (8) | 223 (8) | 253 (8) |
| CLL diagnosis calendar year (Danish/Mayo) | ||||||
| 2002–2007/1993–2007 | 173 (22) | 1961 (30) | 2134 (29) | 120 (34) | 1136 (42) | 1256 (41) |
| 2008–2013 | 282 (35) | 2397 (36) | 2679 (36) | 107 (30) | 766 (28) | 873 (28) |
| 2014–2018/2014–2021 | 347 (43) | 2286 (34) | 2633 (35) | 127 (36) | 813 (30) | 940 (31) |
| In the CLL register | 571 (71) | 4332 (65) | 4903 (66) | - | - | - |
| CLL-IPI available | 329 (41) | 2536 (38) | 2865 (38) | 171 (48) | 1438 (53) | 1609 (52) |
| Low | 165 (50) | 1446 (57) | 1611 (56) | 50 (29) | 507 (35) | 557 (35) |
| Intermediate | 104 (32) | 732 (29) | 836 (29) | 58 (34) | 496 (34) | 550 (34) |
| High | 54 (16) | 295 (12) | 349 (12) | 53 (31) | 359 (25) | 412 (26) |
| Very high | 6 (2) | 63 (2) | 69 (2) | 10 (6) | 76 (5) | 86 (5) |
| Binet stage/Rai stage (Danish/Mayo) | ||||||
| Available | 571 (71) | 4332 (65) | 4903 (66) | 342 (97) | 2673 (98) | 3015 (98) |
| A/0 | 468 (82) | 3523 (81) | 3991 (81) | 128 (37) | 1004 (38) | 1132 (38) |
| B/1 or 2 | 68 (12) | 585 (14) | 653 (13) | 158 (46) | 1414 (53) | 1572 (52) |
| C/3 or 4 | 35 (6) | 224 (5) | 259 (5) | 56 (16) | 255 (9) | 311 (10) |
| IGHV status available | 433 (54) | 3345 (50) | 3778 (51) | 192 (54) | 1634 (60) | 1826 (59) |
| Unmutated | 135 (31) | 1045 (31) | 1180 (31) | 99 (52) | 858 (53) | 957 (52) |
| Mutated | 298 (69) | 2300 (69) | 2598 (69) | 93 (48) | 776 (48) | 869 (48) |
| FISH available | 494 (62) | 3758 (57) | 4252 (57) | 237 (67) | 1913 (70) | 2150 (70) |
| Del(17p) | 28 (6) | 217 (6) | 245 (6) | 14 (6) | 91 (5) | 105 (5) |
| Del(11q) | 32 (6) | 275 (7) | 307 (7) | 26 (11) | 208 (11) | 234 (11) |
| Tri (12) | 83 (17) | 448 (12) | 531 (12) | 40 (17) | 351 (18) | 391 (18) |
| None detected | 140 (28) | 1119 (30) | 1259 (30) | 65 (27) | 483 (25) | 548 (26) |
| Del(13q) | 211 (43) | 1699 (45) | 1920 (45) | 85 (36) | 752 (39) | 837 (29) |
| B2M available | 430 (54) | 3288 (49) | 3728 (50) | 222 (63) | 1796 (66) | 2018 (66) |
| Median (range) | 2.8 (1.0, 31.9) | 2.5 (0.2, 2361.6) | 2.5 (0.2, 2361.6) | |||
| >4.0 mg/L | 98 (23) | 432 (13) | 530 (14) | |||
| ≤4.0 mg/L | 332 (77) | 2856 (87) | 3198 (86) | |||
| Follow-up time, years | ||||||
| Median (IQR/range) (Danish/Mayo) | 3.8 (1.9, 6.2) | 5.0 (2.5, 8.5) | 4.9 (2.4, 8.3) | 4.5 (0, 21.3) | 5.6 (0.0, 25.5) | 5.4 (0.0, 25.5) |
- Abbreviations: IGHV, immunoglobulin heavy-chain variable; IQR, interquartile ranges; T2D, type 2 diabetes.
3.2 Outcomes from the time of CLL diagnosis
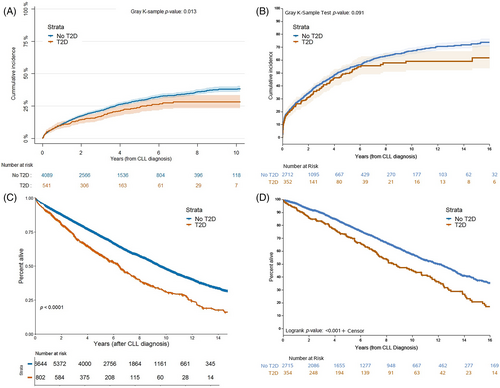
Patients with T2D were less likely to receive treatment for CLL and had a longer TTFT compared with patients without T2D (HR Danish cohort 0.81 [0.67–0.99] and Mayo cohort 0.91 [0.76–1.09]) (Figure 1, Figures 2A, B and Table 2). OS from diagnosis was shorter in patients with T2D than in patients without T2D (HR Danish 1.58 [1.42–1.76] and Mayo 1.29 [1.09–1.52]) (Figures 2C, D, and Table 2). T2D had elevated but nonsignificant risk of death related to CLL (HR Danish 1.15 [0.99–1.34] and Mayo 1.21 [0.92–1.59]) and elevated and significant risk of death unrelated to CLL (HR Danish 2.07 [1.67–2.55] and Mayo 1.78 [1.25–2.54]). Among CLL-related causes of death, T2D had elevated risk of death due to infections in both cohorts (HR Danish 1.25 [1.01–1.55] and Mayo 1.48 [0.74–2.95]) (Table 2). T2D was associated with death due to other non-hematological malignancies in the Danish cohort but not in the Mayo cohort (HR Danish 1.38 [1.02–1.86] and Mayo 1.06 [0.65–1.75]). The associations between T2D and TTFT, OS, and non CLL-related causes of death were independent of CLL-IPI (Table S1).

| Danish cohort | Mayo cohort | |||||
|---|---|---|---|---|---|---|
| Outcome after CLL diagnosis | Events | Univariable HR (95% CI) | Multivariable HR (95% CI) | Events | Univariable HR (95% CI) | Multivariable HR (95% CI) |
| Overall survival | 3167 | 1.63 (1.47–1.81) | 1.58 (1.42–1.76) | 1173 | 1.60 (1.36–1.89) | 1.29 (1.09–1.52) |
| Event-free survival | 1963 | 1.37 (1.24–1.51) | 1.27 (1.16–1.41) | 1898 | 1.17 (1.02–1.34) | 1.07 (0.93–1.23) |
| Time to first treatment | 1220 | 0.78 (0.64–0.95) | 0.81 (0.67–0.99) | 1347 | 0.92 (0.77–1.09) | 0.91 (0.76–1.09) |
| Cause-specific mortality | ||||||
| CLL-related death | 1967 | 1.25 (1.08–1.44) | 1.15 (0.99–1.34) | 578 | 1.22 (0.92–1.61) | 1.21 (0.92–1.59) |
| Due to hematological malignancy | 757 | 0.88 (0.68–1.14) | 0.86 (0.66–1.12) | |||
| Due to CLL malignancy | 382 | 1.21 (0.90–1.64) | 1.16 (0.86–1.56) | |||
| Due to other malignancy | 372 | 1.48 (1.10–1.99) | 1.38 (1.02–1.86) | 137 | 1.07 (0.65–1.76) | 1.06 (0.65–1.75) |
| Due to infection | 838 | 1.32 (1.07–1.63) | 1.25 (1.01–1.55) | 58 | 1.49 (0.75–2.97) | 1.48 (0.74–2.95) |
| Non-CLL-related death | 643 | 2.13 (1.74–2.62) | 2.07 (1.67–2.55) | 193 | 1.79 (1.26–2.56) | 1.78 (1.25–2.54) |
| Unknown cause of death | 402 | 1.02 (0.76–1.38) | 1.02 (0.76–1.37) | |||
- Abbreviations: CI, confidence interval; HR, Hazard ratio.
3.3 Baseline characteristics at first-line treatment for CLL
Collectively in both cohorts, 2839 patients received first-line treatment for CLL during follow-up: 1487 in the Danish cohort and 1352 in the Mayo cohort (Table S2). The median follow-up time from start of treatment was 3.4 and 6.7 years for the Danish and Mayo cohorts, respectively. In the Danish cohort, 164 patients (11%) had T2D and 140 (10%) in the Mayo cohort. The median age was 71 and 65 years, and 64% and 73% were males in the Danish and Mayo cohort, respectively.
Among patients with T2D in the Danish cohort, chlorambucil (Clb) with or without the addition of a CD20 antibody was the most common treatment regimen, followed by bendamustine and rituximab (BR) and fludarabine, cyclophosphamide, and rituximab (FCR) (Table S3). In the Mayo cohort, patients with T2D were also most often treated with Clb with or without the addition of antiCD20 antibody, followed by novel agents, BR, and FCR. Patients receiving Clb-based regimens, novel agents, or BR were older than patients treated with FCR. IGHV unmutated status was most common among patients treated with FCR in the Danish cohort and novel agents in the Mayo cohort.
3.4 Outcomes from first-line treatment for CLL
OS from time of first-line treatment was trending toward being shorter in patients with T2D compared with those without T2D (HR Danish 1.22 [0.95–1.56] and Mayo 1.16 [0.90–1.48]) (Figures S1A, B and Table S4). An inferior or similar but nonsignificant survival trend was observed for patients treated with FCR compared with BR in the both cohorts (HR Danish 2.58 [0.61–10.86] and Mayo 1.13 [0.25–5.06]), also when adjusted for IGHV status and age (Figure S1C, D and Table S5). In the Mayo cohort, patients treated with novel agents had a similar OS compared with BR (HR Mayo 0.67 [0.13–3.39]), while this could not be assessed in the Danish cohort due to low numbers. T2D was not associated with EFS (HR Danish 1.07 [0.75–1.52], data not available for Mayo) (Figure S1E and Table S4).
3.5 T2D characteristics
Patients in the Danish cohort were matched with up to 10 comparator persons without CLL from the general population; data on use of antidiabetic drugs and diabetes-related complications between the two groups patients with T2D are presented in Table S6. Prevalence of T2D was similar in CLL patients (11%) and matched comparators (10%). Use of antidiabetic drugs, number of antidiabetic drugs, and type of antidiabetic drugs used were similar for the two groups. Most patients received one or more antidiabetic drugs (91% and 89% for CLL patients and comparator persons, respectively), and two out of three patients were treated with metformin. The prevalence of macrovascular and microvascular complications was similar across CLL patients and comparator persons. For the Mayo cohort, information on the body mass index was available for 73% of CLL patients with T2D; among these, 90% were overweight or obese.
4 DISCUSSION
In this article, we provided evidence on the survival outcomes of patients with co-occurring CLL and T2D using data from two of the world's largest CLL cohorts. Our findings illustrate a poorer prognosis in patients with T2D compared with patients without T2D, including an inferior overall survival both from CLL diagnosis and upon treatment for CLL. T2D had an elevated risk for increased mortality due to causes of death both related and unrelated to CLL. Moreover, despite the increased risk of death due to CLL, patients with T2D were less likely to receive treatment for CLL. These findings highlight a large subgroup of CLL patients with an inferior prognosis and a possible unmet treatment need.
Previous studies have described a poorer survival in CLL patients with various comorbidities, but to the best of our knowledge, this is the first study describing a shorter overall survival in CLL patients with T2D specifically.6, 7, 13-15, 48 Conflicting results have been published on the association between comorbidity and CLL-related death.6, 12, 15, 48 In the current study, we find that T2D is associated with a poorer survival not only due to causes unrelated- but also due to causes related to CLL. While we present conflicting results on the risk of death due to hematological and non-hematological cancers, findings from both cohorts suggest that the increased risk of death related to CLL is largely driven by death due to infections, though estimates were imprecise for the Mayo cohort. CLL and T2D are both diseases with known inflammatory components and increased risk of infections, as also seen during the SARS-COVID-19 pandemic.49-52 Over the last decades, the risk of death due to CLL-progression has decreased in patients with CLL, whereas death due to infections has proportionally increased and remain the most common cause of death in CLL patients.2 While guidelines recommend pneumococcal and influenza vaccination both for patients with CLL and T2D, and CLL guidelines suggest immunoglobulin replacement treatment for patients with high rates of severe infections and hypogammaglobulinemia, no specific recommendations exist for patients with CLL and concurrent T2D.53-55 Further studies are needed to identify possible interventions that may decrease the risk of severe infections in such patients.
Similar to previous studies on comorbidity, we found that patients with T2D were at increased risk of death due to causes unrelated to CLL.6, 15, 48 We found that patients with CLL and T2D received antidiabetic treatment comparable to patients with T2D without CLL in terms of number and types of drugs. However, it remains unknown if this also correlates to equivalent glycemic control and if glycemic levels significantly impact survival in patients with CLL. Current studies do not suffice to determine if patients with CLL are sufficiently treated for co-occurring T2D or if additional actions are required to optimize glycemic control and prevent diabetes-related complications.56, 57
We have previously described differentiating patterns of treatment timing across different types of comorbidities.14, 15 Despite the increased CLL-related mortality in patients with T2D, T2D tended to have longer time to treatment for CLL and a reduced chance of receiving treatment. While CLL may be detected earlier in patients with T2D due to regular blood work up, our data implies that patients with T2D have similar Binet/Rai stages, CLL-IPI, and absolute lymphocyte count compared with CLL patients without T2D. Thus, it does not seem that a difference in timing of CLL diagnosis explains the timing of CLL treatment. To our knowledge, there is no data supporting a slower progression of CLL in patients with T2D—on the contrary, T2D has been associated with an increased risk of numerous cancers, including CLL.58, 59 It is conceivable that symptoms attributable to CLL (such as anemia and fatigue) may be incorrectly attributed to comorbidities and thus not trigger appropriate treatment of CLL. Moreover, physicians may defer CLL treatment for patients with T2D, despite fulfilling iwCLL criteria for treatment, due to concerns of side effects and toxicity upon treatment. While it is unknown if delayed treatment in patients with comorbidity is associated with a difference in quality of life or shorter survival, it is possible that suspension of treatment may be more prevalent in patients who are ineligible for novel agents compared to patients who are only eligible for chemoimmunotherapy. Our findings suggest a similar overall survival upon novel agent therapy compared with BR in patients with T2D, while patients in the Danish cohort who were not eligible for novel agents had similar or superior overall survival upon BR compared with FCR. While this needs to be confirmed through prospective testing, these findings indicate that BR, possible due to a superior safety profile, may be preferred over FCR in patients with T2D who are not eligible for novel agents.
This study is restricted by its retrospective nature, which limits the inferences that can be made based on these data. In the Mayo cohort, the presence of T2D was self-reported, possible leading to both misclassification and underreporting of T2D. CLL treatment was not randomized but based on physician's choices according to guidelines and thus closely related to prognostic factors and frailty. While we have adjusted for known prognostic factors, the allocation of treatment substantially limits the extrapolation of findings into treatment recommendations. Furthermore, while we have adjusted for differences across patients with and without T2D, there are likely additional dissimilarities we have not been able to account for. As several conditions such as cardiovascular disease, kidney disease, and hypertension may be complications due to T2D and associated metabolic syndrome, we have chosen not to adjust for other comorbidities. The strengths of this study reside in the cohorts being population-based (Danish) or clinic-based (Mayo), thus not excluding CLL patients based on factors such as frailty, age, or geography. Of note, however, the median age at diagnosis in the Mayo cohort was lower than in the Danish cohort suggesting a possible selection bias. As such, the Mayo cohort may not be entirely representative for the US CLL population; however, the similarity to the results found in the Danish cohort suggests that these results are valid also for older patients. Importantly, the analyses have been performed in two different cohorts from Europe and the US with different health care systems; yet, our findings were remarkably consistent across the two populations.
5 CONCLUSIONS
We demonstrate that T2D is associated with a shorter overall survival both from time of CLL diagnosis and from first-line treatment for CLL, while patients with T2D are less likely than patients without T2D to receive treatment for CLL. The increased mortality is largely driven by deaths due to infections. Our findings also indicate that treatment with BR may be preferable to FCR in patients with T2D and that novel agents appear beneficial for this population. The findings of this study emphasize unmet needs and an inferior prognosis in a substantial subgroup of CLL patients with co-occurring T2D. As this patient group is projected to increase considerably in the coming years, additional interventions will be required including awareness of possible under-treatment.
AUTHOR CONTRIBUTIONS
Emelie Curovic Rotbain, Klaus Rostgaard, Caspar Da Cunha-Bang, Henrik Hjalgrim, Henrik Frederiksen, Susan L. Slager, and Carsten Utoft Niemann designed the research; Henrik Hjalgrim, Henrik Frederiksen, Susan L. Slager, and Carsten Utoft Niemann contributed patients; Emelie Curovic Rotbain, Cristine Allmer, Klaus Rostgaard, and Kari G. Rabe analyzed the data; Emelie Curovic Rotbain wrote the first draft of this article; and all authors discussed the results and contributed to the final manuscript.
FUNDING INFORMATION
This research was supported in part by AstraZeneca, CLL-CLUE (ERA-PERMED EU program), and the Danish Cancer Society.
CONFLICT OF INTEREST STATEMENT
Emelie Curovic Rotbain received consultancy fees or travel grants from Abbvie, Janssen, and AstraZeneca. Caspar Da Cunha-Bang received consultancy fees and/or travel grants from AstraZeneca, Abbvie, Janssen, Beigene and Gilead. Carsten Utoft Niemann received funding and/or consultancy fees from Abbvie, AstraZeneca, Janssen, Beigene, Genmab, Takeda, CSL Behring, Octapharma and Eli Lilly outside of this work. Henrik Frederiksen received support outside this work from Alexion, Gilead, Abbvie, Janssen Pharmaceuticals, and Novartis. Henrik Hjalgrim received support from Neye Fonden outside this work. The remaining authors report no conflicts of interest. AstraZeneca was provided with the opportunity to prospectively review the manuscript. The funding sources had no influence on the decision to submit this manuscript, the content of this manuscript, or the interpretation of the data.
Open Research
DATA AVAILABILITY STATEMENT
Data may be made available upon contact to the corresponding author.




