Hemolytic anemia and macrothrombocytopenia: A lipid problem?
A physician or group of physicians considers presentation and evolution of a real clinical case, reacting to clinical information and data (boldface type). This is followed by a discussion/commentary.
1 CASE PRESENTATION
As part of an ongoing IRB approved study of rare and/or difficult to diagnose hemolytic anemias, peripheral blood samples were received from a 19-year-old male college student with a longstanding history of hemolytic anemia accompanied by mildly elevated total bilirubin with indirect hyperbilirubinemia. An ultrasound of his abdomen had revealed a prominent spleen. In addition, the patient had a history of high serum cholesterol, which had not responded to statin treatment. Xanthelasmas were noted on his upper eyelids at the inner corners of his eyes. Blood samples were obtained from the parents and from a healthy volunteer for use as a control.
The combination of longstanding hemolytic anemia and high serum cholesterol which had not responded to statin treatment raised suspicion of sitosterolemia, a rarely diagnosed autosomal recessive disorder caused by homozygous or compound heterozygous variants affecting one of two sterol ATP-binding cassette transporter proteins, ABCG5 (sterolin-1) or ABCG8 (sterolin-2).1 In the intestine, Niemann-Pick C1 Like 1 (NPC1L1) protein transports both cholesterol and plant sterols from the lumen of the intestine into enterocytes. About 50% of cholesterol in the intestinal lumen is absorbed while only about 5% of plant sterols are absorbed, largely due to their return to the intestinal lumen by the heterodimeric ABCG5/ABCG8 transporter in the apical membrane of the enterocyte. Remaining plant sterols that enter the plasma are removed by hepatocytes and transported into the bile via the ABCG5/ABCG8 transporter for excretion. Due to hyperabsorption of plant sterols in the intestine and low biliary excretion, patients with sitosterolemia have extremely high plasma plant sterol levels. Plasma cholesterol levels are frequently elevated as well, especially in children. Clinical manifestations of sitosterolemia may include xanthomas, premature atherosclerosis, hemolytic anemia, and macrothrombocytopenia.2 Splenomegaly is common3 and abnormal bleeding may occur, especially with surgical challenges.4 The hematologic features of the disorder are more common than initially appreciated and may be the presenting symptoms.3
The initial evaluation of the patient's hemolytic anemia was conducted essentially according to our algorithm for laboratory evaluation of patients presenting with symptoms and signs of hemolysis.5 A complete blood count performed with a Siemens Advia 2120i Automated Hematology Analyzer showed: WBC 6.36 × 103μL (ref. 4.5–13); RBC 4.48 × 106μL (ref. 4.4–5.9); Hgb 14.9 g/dL (ref. 13.3–17.7); MCV 101.1 fL (ref. 80–96); MCH 33.2 pg (ref. 26–34); MCHC 32.9 g/dL (ref. 31–36); RDW 14.2% (ref. <= 15.2); platelets 66 × 103/μL (ref. 135–466); MPV 19.8 fL (ref. 7.2–11.8); reticulocytes 2.54% (ref. 0.5–1.5); absolute reticulocyte count (ARC) 113.5 × 103μL (ref. 43–85). Other relevant laboratory findings included a mildly elevated total bilirubin of 2.1 mg/dL (ref. 0.1–1.2) with indirect hyperbilirubinemia, an LDH of 268 U/L (ref. 120–246), and a haptoglobin of 84 mg/dL (ref. 16–200). Wright-Giemsa-stained peripheral blood smears from the patient revealed RBC macrocytosis, the presence of frequent stomatocytes, spherocytes, and platelets which were large and decreased in number (Figure 1A).
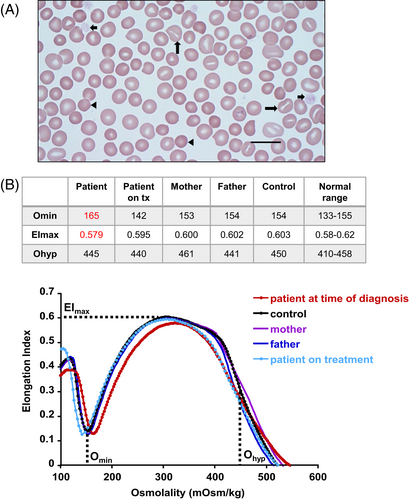
Red blood cells (RBCs) and platelets do not express ABCG5 and ABCG8, rather high plasma levels of plant sterols result in their incorporation into the cell membranes of RBCs and platelets.2 Peripheral blood smears of individuals with sitosterolemia may display stomatocytes and large platelets and sometimes spherocytes and target cells. Plant sterols are C29 and C28 sterols that are structurally similar to cholesterol, a C27 sterol, but differing primarily in the side-chain by the presence of an ethyl or methyl group at the C-24 position, and with or without unsaturation in the steroid rings. Their incorporation into cell membranes tends to be largely restricted to the outer leaflet in contrast to the balanced distribution of cholesterol between both membrane leaflets.6 The conformational shapes of the plant sterols affect the packaging of lipids in lipid bilayers.7 Since plant sterols displace cholesterol in the cell membrane,8 they may also disrupt membrane function by altering membrane fluidity, by disrupting lipid rafts that contribute to the function of membrane proteins, or by disrupting direct cholesterol-protein interactions. Interestingly, after extraction of cholesterol from rabbit RBC membranes with β-cyclodextrin, the RBCs were shown to change from discocytes to stomatocytes.9
In a mouse model of sitosterolemia it has been shown that incorporation of plant sterols into the platelet membrane leads to dysregulation of platelet activation pathways, macrothrombocytopenia, and bleeding.4 In addition to direct effects of plant sterol accumulation in the cell membrane of platelets, there is evidence that plant sterols affect the biogenesis of platelets. Electron microscopy of megakaryocytes from mice with a homozygous Abcg5 mutation and from Abcg5 knockout mice reveals a less well-developed platelet demarcation membrane system that is involved in platelet formation.10, 11
To explore potential alterations in the physical characteristics of the morphologically altered RBCs, we performed ektacytometry on blood samples from the patient and his parents. The parents had normal ektacytometry profiles while the patient's profile indicated an increase in osmotic fragility and slightly decreased deformability resembling mild spherocytosis (Figure 1B).
Ektacytometry, performed on a Laser Assisted Optical Rotational Red Cell Analyzer (Lorrca Maxsis [Lorrca®], Mechatronics Instruments), measures RBC deformability while blood samples are exposed to a constant shear stress and an increasing osmotic gradient.12 An increase in RBC osmotic fragility using standard osmotic fragility tests has been previously reported in individuals with sitosterolemia.3 The increased Omin (i.e., the hypotonic osmolality at which minimum RBC deformability is noted; coincides with osmolality at which 50% of cells hemolyze in an osmotic fragility test)12 of the patient's ektacytometry curve was consistent with this finding. In addition, there was a slight decrease in the elongation index maximum (EImax), indicating a mild decrease in RBC deformability. These findings are consistent with the patient's hemolytic anemia.
Due to the large number of stomatocytes observed in the patient's peripheral blood smear, we performed RBC intracellular cation determinations on blood samples from the patient and his parents. All had normal RBC intracellular cation levels (Table 1).
| K+ | Na+ | K+ + Na+ | |
|---|---|---|---|
| Patient | 284 ± 5.6 | 14.6 ± 0.76 | 298.6 |
| Mother | 286 ± 6.1 | 28.4 ± 0.66 | 314.4 |
| Father | 275 ± 3.8 | 16.9 ± 0.37 | 291.9 |
| Control | 275 ± 4.6 | 30.2 ± 1.30 | 305.2 |
| Ref range | 257.8–334.2 | 12.7–66.1 | 291.1–379.7 |
- Note: Unit of measure mmol/kg Hgb.
The presence of a significant number of stomatocytes in a peripheral blood smear is frequently associated with an RBC hydration defect due to an alteration in the expression or function of one of the membrane channels which control the levels of intracellular monovalent cations.13 In order to rule out a primary erythrocyte hydration defect, we performed RBC intracellular cation determinations by flame emission spectroscopy using a Perkin Elmer AAnalyst 300. RBC intracellular Na+, K+, and Na+ + K+ values were within normal ranges for the patient and his parents, indicating that the presence of stomatocytes in the patient's peripheral blood smears was not a result of a primary RBC hydration defect.
Although a number of ion transport channels have been demonstrated to localize within cholesterol domains, to bind cholesterol, and to show alterations of activity in relation to cholesterol binding,14, 15 the patient demonstrated normal RBC cation levels and evaluation by ektacytometry did not indicate an RBC hydration defect. It is possible that secondary RBC hydration effects could occur among sitosterolemia patients due to displacement of cholesterol from membranes by plant sterols and such effects could vary depending upon which plant sterols are consumed and individual variations in other genes that affect RBC hydration. However, it is clear that the presence of stomatocytes in the peripheral blood smear alone is not sufficient to conclude that a sitosterolemia patient has an RBC hydration defect.
DNA from patient and parent blood samples was sequenced using a 38-gene Hereditary Hemolytic Anemia Next Generation sequencing panel (Agilent SureSelect target enrichment system and Illumina high-throughput sequencing). The sequencing revealed two rare pathogenic variants in the compound heterozygous state in the ABCG8 gene in the patient (c.63_63 + 53del and c.547del). The patient's father was heterozygous for c.63_63 + 53del and his mother was heterozygous for c.547del. Both parents were hematologically normal.
The finding of compound heterozygous ABCG8 pathogenic variants in the patient's DNA is consistent with the autosomal recessive inheritance pattern of sitosterolemia.1 The c. 63_63 + 53del variant is a 54-base deletion over the exon 1 and intron 1 boundary that is expected to disrupt the canonical spice donor site of intron 1, cause intron 1 retention, and result in premature termination. The c.547del variant is a 1-base deletion in exon 4 that causes a frameshift and is expected to result in premature termination. It has been previously reported in compound heterozygous state with another nonsense variant in a Hispanic patient with sitosterolemia.16 Both variants are expected to be targeted to nonsense mediated mRNA decay and result in a loss of function effect. Loss of function is the disease mechanism of this gene for sitosterolemia. Therefore, both variants are interpreted as pathogenic.
Plasma samples from the patient, his parents, and a normal control were analyzed for plant sterols/stanols and cholesterol using gas chromatography with flame ionization detection (GC-FID; Figure 2A). At the time of diagnosis, the patient had highly elevated plasma levels of β-sitosterol + isofucosterol, campesterol, and stigmastanol without an increase in cholesterol. Elevations in plant sterols were not detected in plasma from his parents or the control (Figure 2B).
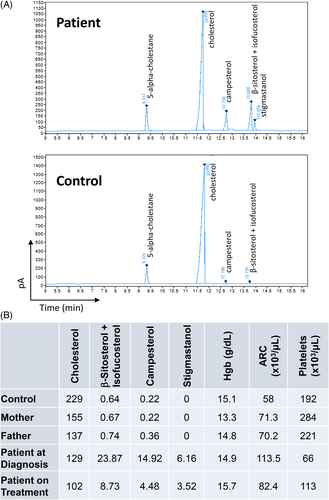
Plant sterols are closely related to cholesterol and assays routinely used to measure cholesterol levels in clinical laboratories are enzyme based and not specific enough to differentiate plant sterols from cholesterol. Plant sterols/stanols and cholesterol extracted from plasma or serum after saponification can be chromatographically resolved and analyzed by gas chromatography–mass spectrometry (GC–MS), by high-performance liquid chromatography-mass spectrometer (LC–MS), or by stand-alone GC with flame ionization detection (GC-FID) as was done in this study. Identification of individual sterols was based upon GC retention times and their mass spectral fragmentation patterns compared with reference standards.17, 18
The patient was started on a low plant sterol and low cholesterol diet and was treated with the NPC1L1 inhibitor ezetimibe at a dosage of 10 mg daily. After 8 months treatment a plasma sample was obtained and concentrations of plant sterols and cholesterol measured by GC-FID. Plant sterol levels were markedly reduced compared with pretreatment levels. Hematologic parameters were also improved including an increase in Hgb, a decrease in ARC which returned to the reference range, and an increase in platelet count (Figures 2B and SS1).
Although plant sterols cannot be completely eliminated from the diet due to their ubiquitous distribution in plants, a low plant sterol diet can be achieved by the avoidance of foods with the highest plant sterol levels. These include vegetable oils, nuts, seeds, whole grains, some fortified foods such as margarine, and certain fruits and vegetables, such as avocado and broccoli. The absorption of residual dietary plant sterols and cholesterol can be reduced by treatment with ezetimibe, an inhibitor of NPC1L1 that transports both plant sterols and cholesterol from the lumen of the intestine into enterocytes.
2 DISCUSSION
Although only approximately 200 cases of sitosterolemia have been reported, there are indications that its prevalence may be greatly underestimated. The incidence of heterozygous loss-of-function mutations in ABCG5 or ABCG8 may be as high as 1 in 220 persons, so that approximately 1 in 200 000 persons may have sitosterolemia due to homozygous or compound heterozygous mutations in either ABCG5 or ABCG8.19 Recently, a case of sitosterolemia caused by double heterozygous mutations in ABCG5 and ABCG8 was reported20 and the incidence of such cases is unknown. Although monoallelic rare polymorphisms and pathogenic variants in either ABCG5 or ABCG8 do not result in sitosterolemia, some have been associated with elevations in plasma plant sterols and/or cholesterol and an increased incidence of atherosclerosis in some individuals. They may also modify other lipid disorders such as familial hypercholesterolemia.21
One probable reason for the under-diagnosis of sitosterolemia is that the hemolytic anemia is often mild and may escape diagnosis in the absence of a thorough hematologic evaluation including reticulocyte count and blood smear evaluation. Delay in diagnosis and misdiagnosis (usually as familial hypercholesterolemia, immune hemolytic anemia, immune thrombocytopenia, or Evans syndrome) are common. In one study, the time to obtain an accurate diagnosis averaged 28.8 years.3
The delay in reaching an accurate diagnosis is particularly problematic because high cholesterol and early atherosclerosis are often detected in children with sitosterolemia. Although both cholesterol and plant sterols may be elevated in sitosterolemia and both have been detected in atherosclerotic lesions, their relative importance in the development of early atherosclerosis has long been a subject of debate. A recent large Mendelian randomization study has provided more substantive evidence that both dietary cholesterol and plant sterols contribute to atherogenesis.22 Significantly, the same study also identified an association between common ABCG5/8 variants and hematologic traits, mean platelet volume, and hemoglobin levels.
It should be noted that although sitosterol is the most abundant plant sterol, it is not always the plant sterol detected at the highest level in individuals with disease-causing ABCG5 or ABCG8 variants. There are more than 60 plant sterols which may be present in various quantities depending on diet23 and it has been shown that they have distinct biological activities. For example, individual plant sterols have been shown to have differential effects on ABC transporter expression, cholesterol efflux, and inflammatory cytokine secretion in cultured models of atherogenic macrophage foam cells.24 Unfortunately, there are currently few clinical laboratories that are able to identify and quantify plant sterols and there is little standardization of assays among laboratories.17
It may be useful to perform platelet function studies when feasible on patients with macrothrombocytopenia and suspected sitosterolemia. Dysregulation of platelet activation pathways, macrothrombocytopenia, and bleeding have been noted in a mouse model of sitosterolemia.4 Examination of plasma from patients with sitosterolemia suggested that the same platelet activation pathways may also be compromised in humans with the disease and abnormal bleeding has been observed in some patients with sitosterolemia.25 It has not yet been determined whether platelet function studies might be useful in the diagnosis and monitoring of sitosterolemia.
A diet low in plant sterols and cholesterol and treatment with the sterol absorption inhibitor ezetimibe are effective in reducing plasma plant sterol levels and improving the major clinical manifestation including the hematologic symptoms in individuals with sitosterolemia.26, 27 One consideration in the use of ezetimibe is that its active form is a glucuronide metabolite, making it less effective in children under 2 years of age due to an immature hepatic glucuronidation system.28 Sitosterolemia does not typically respond to statins as seen in this patient when he was treated with a statin during his teenage years after he was misdiagnosed with hypercholesterolemia. The major target of statins is HMG-CoA reductase which controls a necessary step in the biosynthesis of cholesterol. This enzyme was shown to be markedly downregulated in patients with sitosterolemia, therefore statins are not generally expected to provide an additional benefit.29 If treatment with ezetimibe does not result in sufficient reduction of plasma plant sterol and cholesterol levels, bile acid sequestrants such as cholestyramine may be used to further reduce plant sterol and cholesterol absorption since bile acids are required for the micellar solubilization and absorption of sterols.26 Pharmacological activators for ABCG5/ABCG8 might be expected to be useful in the treatment of sitosterolemia in cases in which residual transporter activity is present, but these have yet to be developed.
In conclusion, an increased awareness among clinicians regarding the signs, symptoms, and treatment of sitosterolemia is necessary to enable early intervention to prevent later complications such as atherosclerosis and the inappropriate and possibly dangerous treatment with corticosteroids and/or splenectomy. In particular, the identification of the combination of hemolytic anemia and macrothrombocytopenia should raise the suspicion of sitosterolemia. Evaluation should include determination of plasma plant sterol levels using appropriate definitive techniques which need to be made more widely available combined with genetic sequencing of the ABCG5 and ABCG8 genes. The relatively recent inclusion of these genes in panels for hereditary hemolytic anemia and platelet disorders has significantly facilitated the detection of sitosterolemia. The identification and study of disease-causing ABCG5 and ABCG8 variants including genotype/phenotype correlations are likely to result in further understanding and suggest new alternative treatments of lipid disorders.
AUTHOR CONTRIBUTIONS
Mary Risinger designed, performed, and analyzed laboratory studies and wrote the manuscript. Phyllis S. Kim and Roberto X. Rodriguez initiated the diagnosis evaluation and supervised the patient's clinical care. Monica Narvaez Rivas performed and analyzed experiments. Kenneth D. R. Setchell, Wenying Zhang, and Theodosia A. Kalfa designed and analyzed diagnostic laboratory studies. Theodosia A. Kalfa is the PI for the Congenital Hemolytic and Dyserythropoietic Anemia study and enrolled the patient and parents. All authors read, edited, and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 1UL1TR001425–01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICT OF INTEREST STATEMENT
Kenneth D. R. Setchell discloses equity in Asklepion Pharmaceuticala and is a consultant to Travere Therapeutics and Mirum Pharmaceuticals. The other authors have no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
The data from this study (anonymized results) are available from the corresponding author upon request.




