Rapid production of clinical-grade SARS-CoV-2 specific T cells
Teck Guan Soh and Yeh Ching Linn contributed equally.
Funding information
The study was funded by a SingHealth Duke-NUS Academic Medicine COVID-19 Rapid Response Research Grant. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abstract
Objectives
To determine whether the frequencies of SARS-CoV-2-specific T cells are sufficiently high in the blood of convalescent donors and whether it is technically feasible to manufacture clinical-grade products overnight for T-cell therapy and assessment of COVID-19 immunity.
Methods
One unit of whole blood or leukapheresis was collected from each donor following standard blood bank practices. The leukocytes were stimulated using overlapping peptides of SARS-CoV-2, covering the immunodominant sequence domains of the S protein and the complete sequence of the N and M proteins. Thereafter, functionally reactive cells were enriched overnight using an automated device capturing IFNγ-secreting cells.
Results
From 1 × 109 leukocytes, a median of 0.98 × 106 (range 0.56-2.95) IFNγ + T cells were produced from each of the six donors, suggesting a high frequency of SARS-CoV-2 reactive T cells in their blood, even though only one donor had severe COVID-19 requiring mechanical ventilation whereas the other five donors had minor symptoms. A median of 57% of the enriched T cells were IFNγ+ (range 20%-74%), with preferential enrichment of CD56+ T cells and effector memory T cells. TCRVβ-spectratyping confirmed distinctively tall oligoclonal peaks in final products. With just six donors, the probability that a recipient would share at least one HLA allele with one of the donors is >88% among Caucasian, >95% among Chinese, >97% among Malay, and >99% among Indian populations.
Conclusions
High frequencies of rapid antigen-reactive T cells were found in convalescent donors, regardless of severity of COVID-19. The feasibility of clinical-grade production of SARS-CoV-2-specific T cells overnight for therapeutics and diagnostics is revealed.
1 INTRODUCTION
A novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of Coronavirus Disease 2019 (COVID-19).1, 2 No specific treatment has been proven to be effective, although promising preliminary data suggested that certain non-specific immunomodulating agents and antivirals can be repurposed to shorten the duration of illness.3-5 While specific vaccines are being developed, passive immunity can be acquired immediately by infusion of plasma from convalescent donors into newly infected patients.6, 7 SARS-CoV-2-specific T cells, which are another key component of adaptive immunity, have not been used therapeutically, primarily because of the concerns of low frequencies of virus-specific T cells, technical feasibility, and long manufacturing time (>1 month).
The aim of this study is to determine whether the frequencies of SARS-CoV-2 specific T cells are sufficiently high in the blood of convalescent donors and whether it is technically feasible to manufacture clinical-grade products overnight for T-cell therapy and assessment of COVID-19 immunity during a fast-progressing pandemic. The specific hypothesis is that SARS-CoV-2 specific T cells can be isolated from the blood of convalescent donors rapidly and efficiently using SARS-CoV-2 specific peptides and a functional selection strategy.
2 METHODS
2.1 Donors
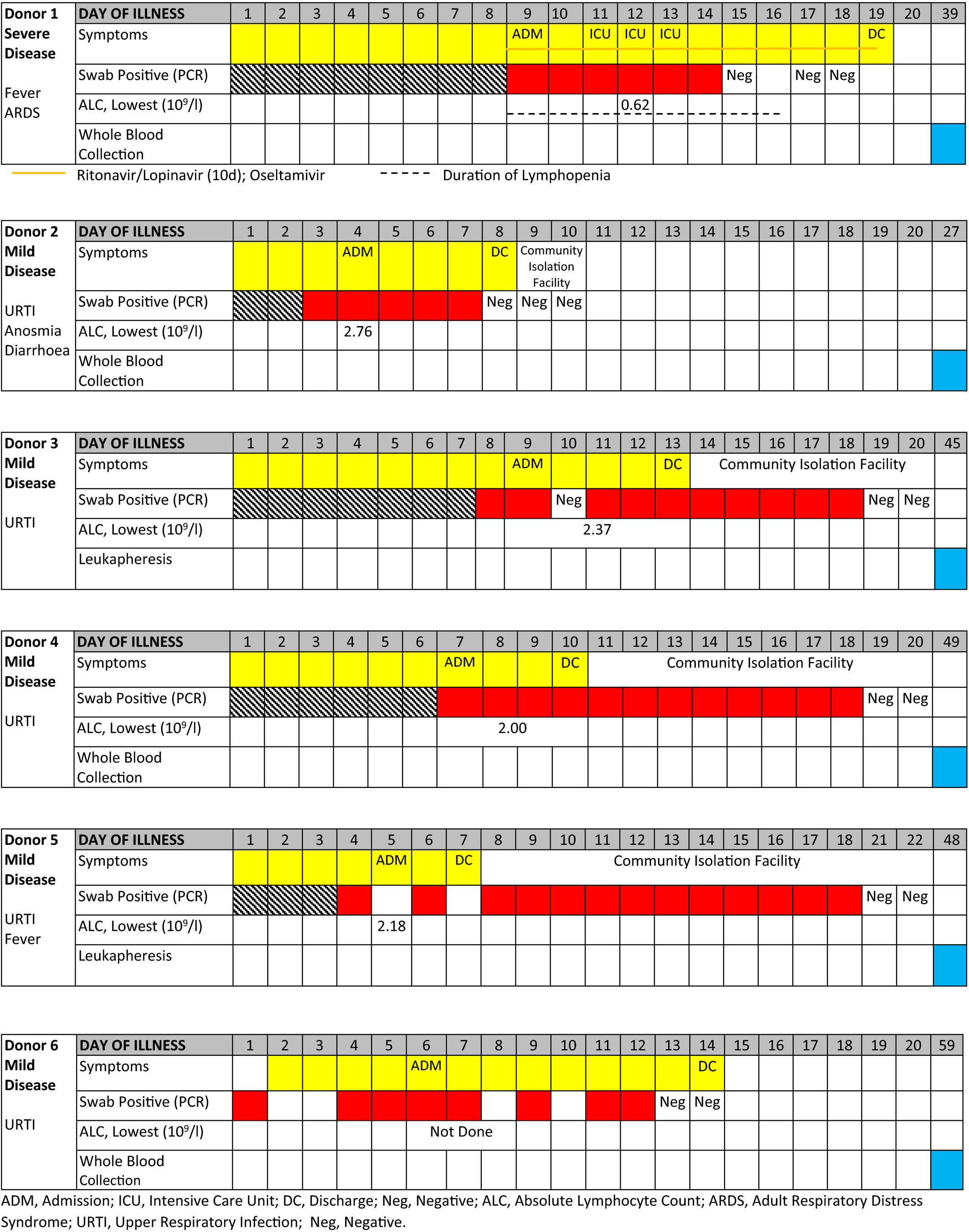
Convalescent donors were referred by their infectious disease physicians. Eligibility criteria included age 21-65, a history of COVID-19 with documented positive test for SARS-CoV-2 in the past but the test had become negative since, fulfillment of all blood donation criteria per standard blood bank practices including a negative blood PCR testing for SARS-CoV-2, and an informed consent with approval by the hospital Institutional Review Board (clinicaltrials.gov NCT04351659). One unit of whole blood or leukapheresis was collected from each donor once. The medical history and type of cells collected from the six donors are summarized in Table 1.
2.2 Cell processing
After plasma extraction and buffy coat preparation (if the collection was whole blood), the leukocytes were processed using the automated CliniMACS Prodigy IFN-γ Cytokine Capture System® (CCS) (Miltenyi Biotec, Germany). Briefly, 1 × 109 cells were stimulated using overlapping peptides of SARS-CoV-2, covering in combination the immunodominant sequence domains of the S protein, and the complete sequence of the N and M proteins (GenBank MN908947.3, Protein QHD43416.1, Protein QHD43423.2, and Protein QHD43419.1). The peptide pools were short 15-mer peptides with 11-amino-acid overlaps, which can bind to MHC class I and class II complexes and thus were able to stimulate both CD4+ and CD8+ T cells. Thereafter, the cells were labeled with the Catchmatrix Reagent containing bispecific antibodies for CD45 and IFN-γ, which was secreted by the stimulated target cells during the secretion period. After the secretion phase, the cell surface-bound IFN-γ was targeted by the Enrichment Reagent, which contained IFN-γ-specific antibody conjugated to superparamagnetic iron dextran particles (MACS® MicroBeads), thus allowing subsequent immunomagnetic cell separation. The unlabeled cells (CCS negative fraction) passed through the built-in magnetic column, whereas the Microbead-labeled cells (CCS positive fraction) were retained in the magnetic field. Afterwards, the magnetic field was turned off and the target cells were eluted into the target cell bag. The entire process took only 12 hours for cell manufacturing.
2.3 Flow cytometry
For the analysis of cell compositions, two antibody panels were used. The first panel contained CD45-VioBlue, CD4-VioGreen, CD3-FITC, Anti-IFN-γ-PE, CD45RO-PEVio770, CD62L-APC, CD8 APCVio770, and 7-AAD for product release and assessment of cell subsets that were IFN-γ positive. The second panel contained CD45-VioBlue, CD4-VioGreen, CD3-FITC, CD16-PE, CD56-PE, CD19-PEVio770, CD14-APC, and CD8-APCVio770 for the assessment of cell content including B cells, NK cells, CD56+ T cells, and CD4:CD8 ratio. Cell acquisition and analyses were performed using the MACSQuant® Analyzer 10 in combination with Express Modes CCS_Purity_h_01 and CCS_Immune_Cell_Composition_h_01 (Miltenyi Biotec, Germany).
2.4 TCRVβ spectratyping
To assess TCR spectra, the CDR3-encoding region of the TCRVβ gene was amplified using 24 TCRVβ subfamily-specific primers and a carboxyfluorescein (FAM)-conjugated TCRVβ constant region-specific primer for the first four clinical products.8 The PCR products were denatured with Hi-Di formamide (Applied Biosystems, Carlsbad, CA) and electrophoresed along with Gene Scan-600 LIZ size standard (Applied Biosystems) on a SeqStudio Genetic Analyzer (Applied Biosystems).
3 RESULTS
3.1 IFNγ+ T-cell yield and functional potency
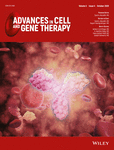
From 1 × 109 leukocytes, containing a median of 0.78 × 109 (range 0.72-0.90) lymphocytes (Table 2), a median of 0.98 × 106 (range 0.56-2.95) IFNγ+ T cells were produced from each of the six donors, suggesting a high frequency of SARS-CoV-2 reactive T cells in their blood, even though only one donor had severe COVID-19 requiring mechanical ventilation whereas the other 5 donors had minor symptoms (Table 1). A median of 57% of the enriched T cells were IFNγ+ (range 20%-74%), indicating their functional competency in rapid IFNγ secretion even with just a brief 4-hour exposure to SARS-CoV-2 peptides (Figure 1A), compatible with a memory recall response.
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Donor 6 | Median | |
|---|---|---|---|---|---|---|---|
| Pre-selection | |||||||
| Starting ALC (109) | 0.73 | 0.72 | 0.87 | 0.82 | 0.90 | 0.74 | 0.78 |
| Lymphocyte subsets | |||||||
| T cells | 86% | 72% | 73% | 77% | 81% | 78% | 77% |
| B cells | 7% | 22% | 20% | 7% | 11% | 9% | 10% |
| NK cells | 7% | 6% | 7% | 16% | 8% | 13% | 8% |
| T cells | |||||||
| CD4 proportion | 65% | 62% | 52% | 56% | 64% | 59% | 60% |
| CD8 proportion | 35% | 38% | 48% | 44% | 36% | 41% | 40% |
| CD56+ T | 3% | 4% | 14% | 9% | 6% | 10% | 7% |
| T cells | |||||||
| Naïve | 23% | 33% | 38% | 36% | 38% | 31% | 35% |
| CM | 35% | 41% | 38% | 45% | 28% | 28% | 36% |
| EM | 36% | 19% | 21% | 17% | 27% | 34% | 24% |
| TEMRA | 6% | 7% | 3% | 2% | 7% | 7% | 7% |
| Product Summary | |||||||
| Cell viability | 96% | 93% | 84% | 92% | 89% | 93% | 92% |
| CD3+ IFNγ+ cells (106) | 1.16 | 0.56 | 0.86 | 2.95 | 1.09 | 0.72 | 0.98 |
| IFNγ+ % in CD3 | 74% | 64% | 38% | 71% | 20% | 50% | 57% |
| Lymphocyte subsets | |||||||
| T cells | 71% | 58% | 45% | 58% | 70% | 33% | 58% |
| B cells | 25% | 38% | 53% | 38% | 27% | 64% | 38% |
| NK cells | 4% | 4% | 2% | 4% | 3% | 3% | 4% |
| T cells | |||||||
| CD4 proportion | 75% | 77% | 63% | 62% | 70% | 73% | 72% |
| CD8 proportion | 25% | 23% | 37% | 38% | 30% | 27% | 28% |
| CD56+ T | 22% | 17% | 11% | 34% | 6% | 28% | 20% |
| CD3+ IFNγ+ cells | |||||||
| Naïve | 0.2% | 1% | 4% | 3% | 4% | 3% | 3% |
| CM | 13% | 17% | 13% | 35% | 12% | 19% | 15% |
| EM | 82% | 70% | 75% | 58% | 75% | 75% | 75% |
| TEMRA | 5% | 12% | 9% | 4% | 9% | 2% | 7% |
| CD4+ IFNγ+ cells | |||||||
| Naïve | 0.3% | 2% | 2% | 3% | 8% | 2% | 2% |
| CM | 10% | 16% | 14% | 30% | 16% | 12% | 15% |
| EM | 89% | 82% | 83% | 68% | 71% | 85% | 82% |
| TEMRA | 0.0% | 1% | 1% | 0% | 5% | 1% | 1% |
| CD8+ IFNγ+ cells | |||||||
| Naïve | 0.2% | 0.8% | 7% | 2% | 1% | 7% | 2% |
| CM | 2% | 11% | 8% | 31% | 18% | 35% | 14% |
| EM | 79% | 33% | 55% | 50% | 51% | 53% | 52% |
| TEMRA | 19% | 56% | 30% | 17% | 30% | 5% | 24% |
3.2 Changes in T-cell compositions before and after enrichment
Before cell enrichment, the median CD4:CD8 ratio was 60:40 and only 7% of the T cells were CD56+ (Table 2). The median CD45RO–CD62L+ T naïve (TN), CD45RO+ CD62L+ central memory (TCM), CD45RO+ CD62L– effector memory (TEM) and CD45RO–CD62L– effector memory RA+ (TEMRA) cell ratio was 35:36:24:7. After cell enrichment, the median CD4:CD8 ratio increased to 72:28, and a substantial proportion of CD56+ T cells (median 20%, range 6%-34%) were observed. The median TN, TCM, TEM and TEMRA ratio in IFNγ+ T cells changed to 3:15:75:7, representing a relative depletion of naïve T cells and enrichment of effector memory cells. Most IFNγ+ CD4+ cells (median 82%, range 68%-89%) were TEM and most IFNγ+ CD8+ cells (median 76%, range 58%-98%) were TEM or TEMRA (Figure 1B). Persistent function and specificity were observed after interval restimulation (Figure 1C).
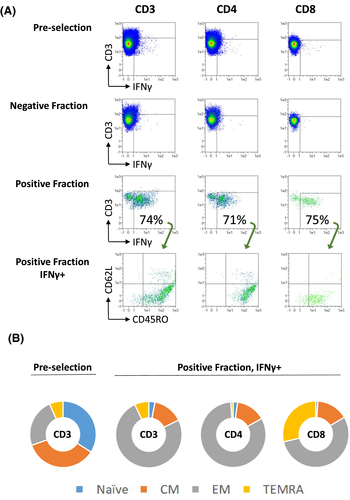
3.3 TCRVβ specificity and purity
Before cell enrichment, polyclonal spectra of TCRVβ in general Gaussian distribution were observed (Figure 2). After enrichment, distinctively tall oligoclonal peaks were observed in the final products, with restricted TCRVβ representations both across subfamilies and within subfamily, suggesting high purity of SARS-CoV-2 specific T cells in general and high-degree of TCR specificity towards SARS-CoV-2 peptides in particular. As expected, the TCRVβ spectra in the negative fraction were similar to those before cell isolation with general Gaussian distribution.
3.4 HLA repertoire
Three of the donors are Chinese Singapore residents, and one each of Caucasian, Indian, and mixed Chinese and European descent. With just six donors, the probability that a recipient would share at least one HLA allele with one of the donors is >88% among Caucasian, >95% among Chinese, >97% among Malay, and >99% among Indian populations (Table S1). The probability of sharing one haplotype could be as high as >15% among Caucasian, Chinese, or Malay residents.
4 DISCUSSION
COVID-19 is a rapidly progressing pandemic with no specific vaccines available thus far. We demonstrated herein for the first time that high frequencies of rapid antigen-reactive T cells were found in convalescent donors, regardless of severity of COVID-19. Clinical-grade SARS-CoV-2 specific T cell production is feasible after a simple blood collection from convalescent donors with the use of SARS-CoV-2 specific peptides and a functional selection strategy, expedient for emergent treatment of severe COVID-19 disease. The manufacturing process is versatile and can produce cells overnight; thus, allowing flexible scheduling to avoid either shortage or wastage of volunteer blood cells. The availability of convalescent donors is naturally proportional to the size of pandemic.
Because the convalescent SARS-CoV-2 specific T cells are allogeneic, bidirectional alloreactivity is a concern. For graft-versus-host disease (GVHD), the enriched cell product is depleted of naïve T cells, thereby limiting the risk of GVHD. In previous studies of HLA-mismatched virus-specific T-cell therapy, GVHD was not a barrier,9-12 as the clinically effective dose was as little as 5x103 virus-specific T cells/kg,12 which was considerably lower than the clinical threshold for GVHD. For the T cells to be effective for virus control, they must act fast before complete donor-cell rejection. In this regard, our cell-selection strategy selects functionally rather than phenotypically and preferentially enriches those T cells that can secrete IFNγ within 4 hours after stimulation with SARS-CoV-2 specific peptides by the nature of memory recall response. In contrast, de novo alloreactivity takes several days to develop in immunocompetent recipients, and could be longer in severely lymphopenic patients with severe COVID-19. In the majority of hospitalized patients with COVID-19, severe lymphopenia developed (lymphocyte count < 0.8 × 109/L), which correlated with disease severity.13-16 Phenotypically, the final cell products are enriched for CD56+ T cells, CD4+ TEM cells, and CD8+ TEM and TEMRA cells, all of which are known to have rapid homing ability to infected tissues and rapid effector function (thus captured preferentially by our functional selection strategy).8, 17, 18
For pandemics caused by novel pathogens, development of specific vaccines for active immunization takes time; however, passive immunity can be acquired immediately via blood component transfusion from recovered patients. Promising preliminary data have been reported on the use of plasma therapy for COVID-19.6, 7 Pharmacokinetically, the level of antibodies universally drops after infusion because of consumption or natural metabolism. In contrast, T cells are a “living drug” and can expand in vivo. Accordingly, a unit of donor's blood may potentially treat more patients and the therapeutic effects of T cells may be more durable than that of plasma therapy. For a dose of 5x103 SARS-CoV-2 T cells/kg used in prior studies,12 one unit of whole blood from each of our donor could generate sufficient cells overnight for 3 to 6 adult recipients and one blood-volume leukapheresis for 20 recipients, without the requirement for ex vivo culture for cell expansion. Pharmacodynamically, SARS-CoV-2 specific T cells may be used alone or synergistically with plasma therapy to establish immediately an adaptive immune status mimicking that after successful vaccination. Under the protection of adaptive immunity, the body will not depend solely on innate immune response, which may contribute to the cytokine storm and SARS pathogenesis.19-21 Coronavirus-specific T cells have been shown to be crucial for virus clearance and may dampen further damage to the lung by the dysregulated, overactive innate immunity mediated by neutrophils, macrophages, and dendritic cells.22-25
For the SARS-CoV-2 specific T cells to be effective, the donor cells must share some HLA with the recipient for proper viral antigen presentation to the donor TCRs. Previous studies have demonstrated that viral-specific T cells from donors who shared only one HLA were efficacious.26 With just six random donors, we estimated that > 88% of Caucasian and > 95% of Asian Singapore residents will share at least one HLA allele with the manufactured cells, suggesting that our approach is feasible for both small-scale and large-scale, off-the-shelf delivery model and manufacture-on-demand model with simple ethnic-group matching.26-28
Extending from therapeutics to diagnostics, our data showing that virus-specific T cells can be detected easily regardless of the severity of COVID-19 suggest that a diagnostic assay can be developed, particularly for patients similar to five of our donors who had mild symptoms and might gone undiagnosed without a history of case contact. By documenting that a sizable population of virus-specific T cells could be isolated in a few hours after just brief stimulation with SARS-CoV-2 specific peptides, our data also support the development of T-cell based immune assays alongside serology to study the biology of viral immunity and impact of public health and therapeutic interventions, as clinical severity and protection might not always correlate with serum antibody levels,29 but with T-cell response instead in some COVID-19 cases.19, 21
This study is limited by small number of donors and lack of recipient data; thus, optimal donor and product characteristics have not been defined, such as medical history and HLA repertoire of the donors, or IFNγ+ purity and CD4:CD8 and TCM:TEM ratio in the cell products. In this regard, the products from Donors 4, 1, and 5 had the highest yield of CD3+ IFNγ+ cells. Donors 4 and 5 were positive for HLA-A2 and their products contained the top two highest amount of CD8+ IFNγ+ cell yield, in line with previous data identifying many SARS-CoV-2-derived antigenic peptides recognized by HLA-A2-restricted cytotoxic T lymphocytes.30, 31 The product from Donor 1 had the highest purity of IFNγ+ cells and highest frequency of rapid-acting and -inflamed tissue-homing TEM cells. This donor had the worse symptoms and lymphopenia during COVID-19 and had the most robust rebound of T cells at the time of blood donation, having the highest proportion of T cells (86%) in lymphocyte subsets and highest TEM (36%) before cell selection among all donors. Collectively, our preliminary data suggest that certain donor factors could affect cell yield and composition, which might in turn affect clinical outcome after adoptive transfer. Further investigations of these hypotheses in future clinical trials are warranted.
4.1 Clinical implications
Fundamental first proof-of-principle data are provided herein demonstrating that it is feasible to produce clinical-grade virus-specific T cells overnight for urgent treatment of novel pathogens. This study, therefore, opens a brand new avenue for T-cell based management and immunity assessment for the current and future emerging infectious diseases. Based on this development, multinational clinical trials of adoptive transfer of similarly-prepared SARS-CoV-2 specific T cells from convalescent donors to patients with COVID-19 are underway (clinicaltrials.gov NCT04457726).
5 CONTRIBUTORS
WL designed the study. TGS and LKT performed the cell manufacturing. YCL, LKT, and MSS prepared the cell collection and clinical summary. YCL, JGL, JL, WJC, LPK, MLP, TTT, LKT, and MSS participated in donor evaluation and eligibility screen. MC performed HLA analyses. KPN and KCH did the TCR typing. MSS coordinated regulatory affairs and activities across all sites. WL drafted the manuscript. YCL, TGS, LKT, and MSS revised the manuscript. All authors participated in the interpretation of data, revision of the manuscript, and approval of manuscript submission.
6 ETHICS STATEMENT
This protocol has been reviewed by the SingHealth Centralised Institutional Review Board for ethics approval. This approval is mutually recognized by National Healthcare Group Domain Specific Review Board (DSRB).
ACKNOWLEDGMENTS
We thank the donors for their blood donation; the staff at Singapore General Hospital Haematology Center, Health Sciences Authority HLA Lab, and National Cancer Institute Singapore Apheresis Centre for technical supports; Goh Foundation and Children's Cancer Foundation for programme support; and Kee Chong Ng, Alex Tiong-Heng Sia, Yoke Hwee Chan, Germaine Liew, Shui Yen Soh, Ah Moy Tan, Kai-Qian Kam, Queenie Gan, Wilson Low and the staff at KK Research Centre for their administrative supports.
CONFLICT OF INTEREST
WL is a part-time advisor to Miltenyi Biomedicine.