Small extracellular vesicles: Yields, functionalization and applications in diabetic wound management
Abstract
Diabetic wounds have imposed a significant burden on both patients and society with prolonged healing processes hindered by dysfunctional skin repair cells. Small extracellular vesicles (sEVs), as the important media for intercellular communications, show promising therapeutic potential in treating diabetic wounds by restoring cellular functions. However, low yields and limited bio-function of sEVs greatly challenge their large-scale clinical use. Here, we briefly overview the biogenesis and cellular uptake of sEVs, emphasize current advances in improving the yields of sEVs and optimizing the function of sEVs with engineering approaches, and summarize the applications of engineered sEVs in diabetic wound treatment. Furthermore, the undissolved issues during the clinical transformation of engineered sEVs are also discussed. This critical review aims to provide meaningful guidance for future applications of engineered sEVs in the management of diabetic wounds.
1 INTRODUCTION
As a typical representative of chronic wounds, diabetic wounds have been recognized as a critical clinical issue over the past few decades, besetting the medical community worldwide, specifically with the coming of the aging society.1 Generally, repetitive infection, excessive inflammatory response, and impaired vascular regeneration are recognized as the characteristic pathological features of diabetic wounds.2-4 The high glucose and toxic advanced glycation end-products (AGEs) cause diabetic wounds to deviate from normal healing processes, including hemostasis, inflammation, proliferation, and remodeling.5 Basically, behavior dysregulation of skin repair cells, such as immune cells, fibroblasts, epidermal cells, and vascular endothelial cells, at wound beds is considered to suspend the repair process.6 For example, overactivated immune cells secret too much inflammatory cytokines, which further reduces collagen deposition, impairs re-epithelization and vascular maturation.7 Therefore, developing effective strategies to regulate and restore normal cellular behaviors is expected to recover the healing processes of diabetic wounds from the pathogenesis.
Small extracellular vesicles (sEVs) refer to a heterogeneous group of small, lipid-bound nanoparticles, and are classified into natural sEVs, engineered sEVs and sEV-inspired nanovesicles (NVs) based on the origin of their constituents.8 Of note, a lot of literatures have embraced the term “exosomes”, referring to those of endosomal origin characterized by a diameter spanning from 40 to 160 nm.9 While sEVs are operational terms considering the size of EVs with a diameter of less than 200 nm, which might be preferable according to Standard launched by Li et al.10 Thus, we adopt the term “sEVs” to collectively denote both small EVs and exosomes. Natural sEVs were first discovered in reticulocytes in 198311 and were subsequently found to be secreted by almost all types of mammalian cells.12 Characteristic analysis demonstrates that natural sEVs are nanosized (40–200 nm in diameter) double-membrane vesicles containing DNAs, RNAs, proteins, lipids, metabolites, and cytosolic molecules.9, 10 Based on these characteristics, natural sEVs are endowed with many functions, such as mediating intercellular communication,13 desirable drug delivery,14, 15 presentation of antigens,16 and therapeutic tools,17 promising to be powerful repair and delivery tools.
As the biomolecule delivery systems, sEVs have displayed several noticeable advantages including less immunogenic and biocompatible,18 sheltering genetic cargoes from degradation,19 crossing blood-brain barrier,20 and suitable for long circulation in vivo.21 As effective therapeutic tools, sEVs have been demonstrated to facilitate diabetic wound healing by modulating the cellular behaviors to optimize inflammatory response, angiogenesis, re-epithelization, and scarless healing.22 For instance, we previously found that sEVs from mesenchymal stem cells (MSCs) accelerated angiogenesis and collagen deposition in diabetic wounds of mice, exerting positive effects on wound healing.23, 24
However, inefficient therapeutic cargoes, low yields, and high production costs limit the clinical translation of natural sEVs. Researchers have attempted to improve the yields25-27 and functionalize sEVs28, 29 to remove these barricades and promote clinical translation.30 Herein, we overview the biogenesis and uptake of sEVs, the methods to increase the yields of sEVs and obtain functionalized sEVs as well as specific applications of engineered sEVs in diabetic wound management. In addition, the concerns and prospects about employing engineered sEVs are discussed. We hope it could help to improve the current understanding of engineered sEVs in the management of diabetic wounds.
2 BIOGENESIS AND UPTAKE OF sEVs
For the biogenesis of sEVs (Figure 1), fluid and extracellular components, such as proteins, lipids, ions, molecules, and cell surface proteins, enter cells via plasma membrane invagination and endocytosis (Figure 1A).9 Subsequently, the plasma membrane bud formation process in the cellular luminal side presents with an outside-in orientation, leading to the generation of early sorting endosomes (ESEs, Figure 1B).31 Next, a part of the ESEs can fuse with the contents of the endoplasmic reticulum (ER), mitochondria, and trans-Golgi network (TGN). After fusing, ESEs grow into late sorting endosomes (LSEs), where a second invagination takes place to form intraluminal vesicles (ILVs, precursor of sEVs).32 During this process, cytoplasmic constituents enter into these newly formed ILVs. Meanwhile, the proteins that are originally located on the cell surface can also be distinctly incorporated into ILVs (future sEVs) with different sizes (Figure 1C). LSEs then give rise to intracellular MVBs with more ILVs (Figure 1D).32 Finally, some MVBs undergo degradation by fusing with lysosomes or autophagosomes, while others are conveyed to the plasma membrane for exocytosis (Figure 1E), leading to the release of sEVs (Figure 1F).33 A collection of proteins is involved in the biogenesis of sEVs, such as the endosomal sorting complex required for transport (ESCRT) proteins and some markers for sEVs.9 As mentioned, sEVs are endosome-sourced nanovesicles with a diameter of no more than 200 nm.10 In contrast, apoptotic bodies, another type of EVs, emanate from dying cells and encompass sizes ranging from 50 to 5000 nm in diameter, typically tending towards the larger end of the range.34
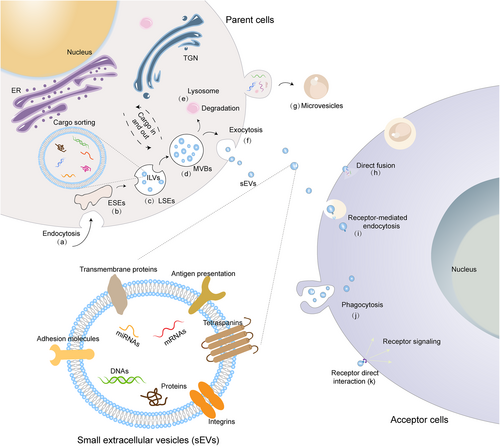
Biogenesis and uptake of sEVs. (A) The process of invagination and endocytosis. (B and C), The formation of ESEs (B) and ILVs (C). (D) The transition from LSEs to MVBs. (E) The degradation of MVBs inside lysosomes. (F) Exocytosis and release of sEVs. (G) The formation of microvesicles. sEVs are ingested by acceptor cells via direct fusion (H) Clathrin-mediated endocytosis (I) phagocytosis (J) and receptor (direct) interaction (k).
When approaching recipient cells closely, sEVs are reported to interact with them via several routes including membrane fusion, internalization, and receptor (direct) interaction.13, 35 Specifically, membrane fusion depends on lipid contents on the surface of sEVs and receptor cells. Their membranes straightforwardly fuse together and the intraluminal cargoes of sEVs are released into the cytosol of the receptor cells. Then, the hydrophobic lipid bilayers of sEVs and cells enlarge to shape into one single coherent structure (Figure 1H).35 For internalization, sEVs hang onto receptor cells, dock with the plasma membrane of the latter, and then get internalized by surface receptor activation (Figure 1I).31 Clathrin-mediated endocytosis,36 phagocytosis,37 and macropinocytosis38 are internalized routes of sEVs (Figure 1J). Besides, during receptor (direct) interaction, transmembrane proteins of sEVs bind directly to proteasomes of the cell membrane to induce downstream signaling cascades and release soluble ligands (Figure 1K). Hence, the contents of sEVs are selectively absorbed into the receptor cells.13 However, mechanisms for the different routes of sEV uptake by acceptor cells and the distinct fates (localization and degradation) of sEV cargo delivery remain incompletely characterized.
3 IMPROVING THE YIELDS OF sEVs
Because of their ability to transport bioactive agents to target cells efficiently, sEVs offer promising therapeutic potential for the treatment of diabetic wounds. However, challenges related to large-scale production and functional improvement of sEVs may limit their clinical use.39-41 In this section, we overviewed current strategies to achieve the large production of sEVs (Figure 2).
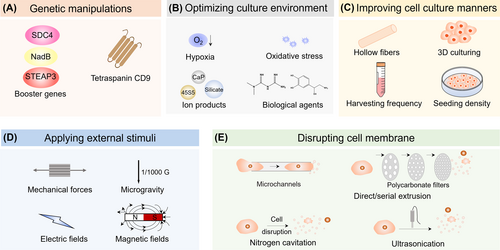
Several manipulating methods of parent cells for increasing the yields of sEVs. These methods are classified into five categories, including genetic manipulations (A), optimizing culture environment (B), improving cell culture manners (C), applying external stimuli (D), and disrupting cell membrane (E).
3.1 Genetic manipulation of parent cells
The overexpression or knockdown of regulatory proteins involved in the biogenesis of sEVs or cellular metabolism of parent cells via genetic manipulation is supposed to improve their yields (Figure 2A). Kojima et al. executed a genetic approach by co-expressing three key proteins, namely STEAP3 (involved in sEV biogenesis), syndecan-4 (facilitating MVB formation), and L-aspartate oxidase (augmenting cellular metabolism), in patient-derived human MSCs (hMSCs) and HEK293T cells.42 The yield of released sEVs was increased by at least 15 folds. Additionally, the overexpression of tetraspanin CD9 substantially amplified sEV quantity from HEK293 cells, as CD9 is closely linked to cell motility, adhesion, and fusion.43 These boosters provide a potential solution to large-dose requirement of HEK293 cells or hMSCs derived sEVs during clinical applications.
3.2 Optimizing culture environment
Optimizing the culture environment can affect the cellular metabolic behaviors of parent cells and improve the secretion of sEVs. Several manners were summarized below (Figure 2B).
Hypoxia: Hypoxia has been observed to boost the secretion of sEVs, which may be mediated by hypoxia-inducible factor-1α (HIF-1α).44 For human umbilical cord MSCs (hucMSCs), 5% O2 hypoxic environment boosted the secretion of sEVs and the yield of sEVs was increased by about 90%, which might be attributed to the enhanced proliferation rate and viability of hucMSCs.45 Hypoxic environments of 1% and 0.5% O2 have been observed to stimulate higher sEV secretion from bone marrow-derived MSCs (BMMSCs), resulting in an approximate doubling of sEV yield following stimulation.46-48 Importantly, hypoxia-primed parent cells not only enhanced sEV yields but also modulated the therapeutic contents of the sEVs, particularly those with angiogenic and anti-inflammatory properties, thereby offering potential benefits for the management of diabetic wounds.
Oxidative stress: Inducing oxidative stress in parent cells through specific culture conditions presents another avenue to amplify sEV yields. Reportedly, the treatments of 80 nM ethanol49 or 600 μM H2O2 50 boosted the section of sEVs from retinal pigment epithelium cells (ARPE-19). Moreover, the sEVs secreted under these conditions exhibited heightened expression of vascular endothelial growth factor receptors (VEGFR) on their membranes, along with increased VEGFR mRNA within their lumens. Consequently, the resulting sEVs demonstrated enhanced capacity to stimulate tube formation by human umbilical vein endothelial cells (HUVECs) in vitro, a process critical for angiogenesis during the healing of diabetic wounds.
Ion products: A wide array of ion products has been explored for their potential to modulate parent cell metabolism and sEV biogenesis. For instance, treatment of macrophages (RAW264.7 cells) or monocytes (THP-1 cells) with calcium phosphate nanoparticles (CaP NPs) at a concentration of 500 μg/mL resulted in approximately a 2-fold increase in the secretion of sEVs.51 This effect can be attributed to intracellular Ca2+ inducing membrane lipid distribution and compromising the cytoskeletal integrity of cells.52 The application of 45S5 Bioglass® (BG) induced an upregulation in the expression of neutral sphingomyelinase-2 (nSMase2) and Rab27a in MSCs, thereby enhancing the nSMases and Rab GTPases pathways and leading to a nearly 3-fold enhancement in sEV yield after 72 h culturing.53 Stimulation of endothelial progenitor cells (EPCs) with a 1/128 dilution of a silicate ion solution resulted in approximately a 2-fold improvement in the particle concentration of derived sEVs, accompanied by an upregulation of proangiogenic factors.54
Biological agents: Incubating hBMMSCs with metformin (1 mM) for 24 h, the production of hBMMSC-sEVs was enhanced by about 2 folds because metformin was the activator of AMP-activated protein kinase (AMPK) and it boosted the release of sEVs through an autophagy-related pathway, accompanied with the phosphorylation of synaptosome-associated protein 29.55 The combination of 100 μM norepinephrine and N-methyldopamine enhanced the metabolic activity of hBMMSCs and achieved about 3-fold enhancement on the secretion of sEVs because N-Methyldopamine and norepinephrine could bind to dopamine and adrenergic receptors to increase cellular metabolism.56
As discussed above, a range of strategies centered around optimizing the culture environment have proven effective in enhancing the secretion of sEVs from diverse parent cell types, including MSCs, macrophages, and endothelial progenitor cells. Importantly, these strategies hold significant potential for advancing sEV-based approaches in the management of diabetic wound healing due to the high-dose demands and the amplified angiogenic and anti-inflammatory properties exhibited by sEVs under these optimized conditions.
3.3 Improving cell culture manners
Seeding parent cells on the hollow-fiber bioreactor culture system can improve the yields of sEVs by sustaining a large number of cells and producing highly concentrated cell culture supernatants (Figure 2C).57 For example, by culturing HEK293 cells on the hollow-fiber (molecular weight cut-off of 20 kDa) with 20 mL serum-free medium and collecting conditioned medium three times per week, the yield of sEVs was improved to approximately 40 folds compared to conventional flasks.57 Haraszti et al. combined microcarrier-based 3D cell culture with tangential flow filtration concentration technique (TFF, a technology to isolate sEVs according to size) to achieve high yields of sEVs.25 The number of sEVs harvested from 3D-cultured MSCs was more than 20 times than that from 2D culture. Furthermore, the yield of sEVs could be improved to about 140 folds by combining 3D culture with TFF.
High frequent collection and low seeding density of the parent cells are beneficial to the yields of sEVs. Patel et al. showed that decreasing the seeding density of BMMSCs from 1 × 104 cells/cm2 to 1 × 102 cells/cm2 increased the number of sEVs by about 100 folds (passage 2) and 50 folds (passage 5).58 Similar trends were observed with human dermal microvascular endothelial cells (HDMECs), HEK293T cells, and HUVECs. They also observed that increasing collection frequency improved the yield of sEVs properly to about 2.6 folds (1 × 104 cells/cm2, every 12 h) compared to those collected every 24 h. These unique phenomena might be attributed to increased metabolic effects and decreased cell–cell contacts, as well as increased total available cell membrane surface area.58 In summary, optimizing parent cell culture methodologies significantly enhances sEV secretion without altering their functions, theoretically satisfying the requirements of high-dosage sEVs.
3.4 Applying external stimuli
In addition to optimizing culture environment of parent cells, applying specific external stimuli is also believed to improve the yields of sEVs, as demonstrated in Figure 2D.
Mechanical forces: MSCs were cultured on a Fibra-Cel scaffold and stimulated with a culture-medium flow of 0.5 mL/min. The production of sEVs from these cells was improved by about 40.7 and 3.4 folds than that in the 2D and 3D static counterparts, respectively. Besides, skeletal muscle cells (SkMCs) seeded on a polydimethylsiloxane (PDMS) elastic scaffold undergoing mechanical stretching with 25% strain cyclic stretch (1 Hz) could produce about 11-fold higher sEV-yield than those without undergoing mechanical stretching. The mechanosensitivity of yes-associated protein (YAP) was speculated to participate in the production of sEVs.59
Electric fields: The stimuli of electric fields were believed to accelerate the secretion of sEVs through the activation of Rho GTPase60 or ESCRT pathway. After stimulation of murine fibroblast cells (3T3 Swiss Albino cells) with a constant current low-level electric field (0.34 mA/cm2) for about 1 h, the number of sEVs secreted by above cells was enhanced by 1.7 and 1.26 folds, respectively.60
Others: Magnetic fields, and microgravity were also explored to boost the generation of sEVs. According to an open patent, applying the magnetic force between 0.3–1 T to the positively charged polymer magnetic nanoparticle pre-treated MSCs induced the generation of sEVs with the diameter of 91–169 nm. Regrettably, the exact increase in the yields of sEVs was not revealed (patent WO2021086139). Another frontier study showed that culturing MSCs under a microgravity microenvironment (1/1000 G) increased the number of sEVs by about 10% (patent WO2021162114).
3.5 Disrupting cell membrane
Disrupting the cell membrane of parent cells generated another sEVs called sEV-inspired nanovesicles (NVs), which showed similar compositions, sizes, and cellular communication, yet significantly-improved yields in comparison with their naturally derived counterparts. Therefore, fabricating NVs may be an alternative strategy to obtain biomimetic sEVs with elevated yields, homogeneity, and purity via simple procedures (Figure 2E).
Extrusion: Extruding cells through microchannels or filters with diminished micro-sized pores were employed to fabricate biomimetic NVs. Jo et al. reported the first example of biomimetic NVs by extruding murine ESCs through the microchannels.61 The characteristics of generated NVs were influenced by wettability and geometry as well as the flow rate of the microchannels. Besides, the serial extrusion strategy that forces cells to pass through a series of filters with successively-decreased pore sizes was also employed to cleavage cells into NVs.62 Lee et al. co-cultured BMMSCs with iron oxide NPs (IONPs) for 24 h and extruded IONP-BMMSCs through 10- and 5-μm and 400-nm pore-sized PC membrane filters with a mini extruder, sequentially. The obtained NVs contained 2.0–2.5 folds of bioactive contents than natural sEVs from IONP-BMMSCs.62
Nitrogen cavitation: Subjecting cells to nitrogen gas-induced cavitation forces at 0 °C led to the destruction of cells via expanding bubbles, releasing cellular components into the fluid and forming membrane-derived NVs.63 By this mean, NVs were obtained with a significantly increased production yield (approximately 16-fold higher). Although these NVs contained fewer subcellular organelles and genetic cargoes, they exhibited higher levels of targeting ligands. Recently, large-dose cholesterol-embedded NVs that co-loaded with antibiotics and anti-inflammatory agents were fabricated from activated neutrophils. This formulation demonstrated efficient targeting to the lung vasculature, mitigating lung bacterial infection and inflammation.64 Therefore, this method holds promise for utilizing NVs as an advanced delivery system with high targeting efficiency.
Others: Ultrasonication and alkaline treatment were also used to fabricate NVs. Wang et al. sheared the intact hucMSCs into NVs with comparative therapeutical effects by ultrasonicating cells for 60 s. The yield and the production rate as well as the cost were improved by 20 folds, 100 folds and decreased to 10%, respectively. Furthermore, the NVs showed similar promoting effect on the proliferation and migration of dermal fibroblasts.65 As for the treatment of alkaline solution with the combination of sonication and ultracentrifugation, it broke and disintegrated U937 cells into membrane sheets and unloaded unwanted cytosolic proteins and nuclear cargoes from the cells. Next, the generated membrane sheets were treated with pH neutralization, followed by sonication and buoyant density gradient ultracentrifugation. The generated biomimetic NVs showcased comparable features (i.e., diameter, morphology, and biomarkers) but absent unwanted luminal cargoes and improved yield (200 folds).66
3.6 The effect of improving yield on the function of sEVs
Indeed, the therapeutic efficacy of sEVs is predominantly determined by both their internal cargoes and surface ligands. The impact of strategies aimed at enhancing sEV yield on their function varies based on the underlying mechanisms, necessitating a case-by-case analysis. For example, strategies involving the modulation of regulatory proteins associated with sEV biogenesis and the improvement of cell culture conditions are expected to have limited influence on sEV function, as these approaches generally do not alter the surface ligands or internal contents of the sEVs, at least theoretically.
In contrast, alterations in the culture environment have the potential to influence sEV functions by affecting the composition and types of cargoes present. For example, the application of hypoxia-pretreatment not only leads to increased yields of MSC-derived sEVs but also augments the content of angiogenic and anti-inflammatory cargoes, encompassing miRNAs and proteins.44-48 Culturing ARPE-19 in culture medium containing ethanol49 or 600 μM H2O2 50 was observed to upregulate the expression of VEGFR on the membrane and more VEGFR mRNA in the lumen of sEVs, thus affecting the angiogenesis of obtained sEVs. Such alterations affect the angiogenic potential of the resulting sEVs. The ion products derived from BG enhanced both sEV production from MSCs and their angiogenic function. This is achieved by activating nSMases and Rab GTPases pathways within MSCs and elevating microRNA-1290 levels within sEVs.53 A similar phenomenon has been observed following the pre-treatment of EPCs with a silicate ion solution.54 In summary, strategies that modify the culture environment are more likely to influence the functions of sEVs by affecting their cargo composition and species, thereby providing a means to tailor sEV properties for specific therapeutic applications.
The impact of applying external stimuli on the function of sEVs does indeed exhibit variations depending on the specific method employed. For instance, the application of mechanical force to parent cells not only increased the secretion of DPSC-derived sEVs but also enhanced their function through YAP-mediated mechanosensing and heightened Wnt signaling.59 Conversely, culturing murine fibroblast cells under a low-level electric field primarily led to an increase in sEV secretion, with limited discernible changes in sEV function.60
In the realm of NV fabrication, the strategies based on extrusion61 significantly increased the yield of NVs while showed limited changes in sEV function. Nitrogen cavitation,63, 64 ultrasonication,65 and treatment with alkaline solution66 have been proposed as the methods to generate NVs with reduced subcellular organelles and genetic cargoes but enriched targeting ligands and improved production yields. NVs produced through these techniques are well-suited for constructing drug delivery systems with a high targeting efficacy.
Collectively, the summarized approaches for enhancing sEV yields hold the potential to advance the clinical application of high-dose sEVs in diabetic wound management. However, further systematic studies are warranted to thoroughly explore the extent to which these methods influence the biofunction of sEVs, especially when aiming to increase their yields.
4 OPTIMIZING THE FUNCTION OF sEVs
Native sEVs inherently possess limited therapeutic components or targeting capabilities, thereby constraining their full therapeutic potential. Addressing these limitations calls for the functionalization of sEVs on-demand. This functionalization process can be broadly categorized into two approaches: endogenously manipulating parent cells (as depicted in Figure 3) or exogenously modifying isolated sEVs (as illustrated in Figure 4, Tables 1 and 2).
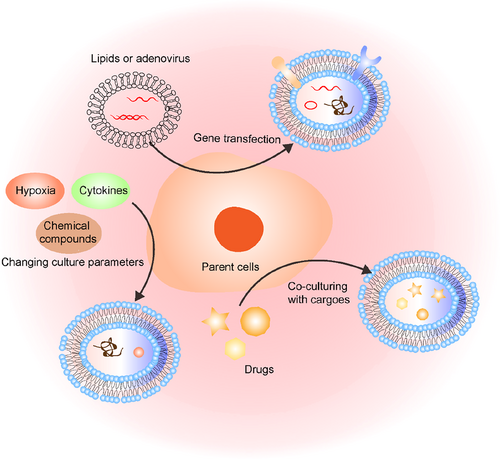
Manipulation methods of parent cells to functionalize sEVs, including gene transfection, co-culturing with cargoes, stimulating parent cells with cytokines, hypoxic environments, and chemical compounds.
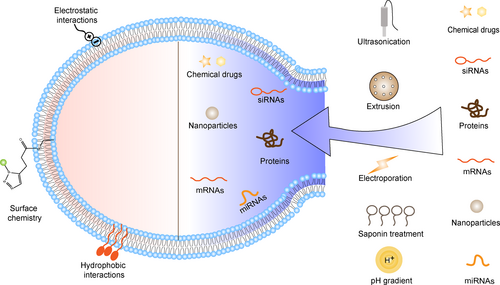
Functionalizing methods of isolated sEVs are classified into surface and internal modifications. The surface of isolated sEVs can be modified through surface chemistry, electrostatic or hydrophobic interactions. The internal contents including chemical drugs, siRNAs, proteins, nanoparticles, mRNAs, and miRNAs could be encapsuled into the isolated sEVs through ultrasonication, extrusion, electroporation, saponin treatment, and pH gradient.
| Engineering approaches | Cargo characteristics | Technical processes | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| Surface chemistry | SDF-1 protein | 1. Copper-free click chemistry and specific technical process was not provided. | High reaction rate, selectivity and yields. | May change activities of membrane proteins on sEVs. | 67 |
| AA | 1. Adding DSPE-PEG-SH to sEVs under rotation (4 °C, 20 min); | 68 | |||
| 2. Adding HAuCl4 to the mixture (37 °C, 40 min); | |||||
| 3. Ultracentrifugation (100,000 g, 30 min) to remove residual DSPE-PEG-SH and HAuCl4; | |||||
| 4. Adding the mixture of NaBH4 and AA to initiate the reduction reaction and stirring for 5 min gently. | |||||
| Pd-Pt nanosheets | 1. Activating the -COOH groups by adding EDC and NHS to Pd-Pt nanosheets dispersed MES buffer (RT, 2 h); | 69 | |||
| 2. Adding sEVs to the mixture and shaking overnight; | |||||
| 3. Harvesting sEV-Pd-Pt by ultracentrifugation (8000 g, 20 min). | |||||
| Hydrophobic interactions | HsiRNAs | 1. Synthesizing oligonucleotides; | Ultrahigh loading efficacy for siRNAs with the assistance of cholesterol conjugation strategy. | HsiRNAs grafted on the surface of sEVs is easily degraded in vivo. | 70 |
| 2. Co-incubating given numbers of sEVs and known amounts of hsiRNAs (37 °C, 1 h); | |||||
| 3. Centrifuging above mixture (100,000 g, 90 min) to remove unloaded hsiRNAs. | |||||
| PBP | 1. Dissolving Cy5.5-labeled DMPE-PEG-PBP in PBS solution; | 71 | |||
| 2. Co-incubating Cy5.5-labeled DMPE-PEG-PBP and sEVs (25 °C, 30 min); | |||||
| 3. Washing out excess Cy5.5-labeled DMPE-PEG-PBP via ultrafiltration. | |||||
| Electrostatic interactions | FPG3 | 1. Mixing FPG3 and sEVs (RT, 20 min); | High efficacy for cargoes with positive potentials. | May shorten long circulation time of sEVs. | 72 |
| 2. Ultrafiltering the mixture with 300 K Nanosep. |
- Abbreviations: AA, ascorbic acid; EDC, 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; FPG3, Fluorinated peptide dendrimers; MES, 4-morpholine ethanesulfonic acid; NHS, N-Hydroxysuccinimide; PBP, P-selectin binding peptide; RT, room temperature; SDF-1, stromal cell-derived factor 1; sEVs, small extracellular vesicles; siRNAs, small interfering RNAs; ssDNAs, single-stranded DNAs.
| Engineering approaches | Cargo characteristics | Technical processes | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| Ultrasonication | Ag NPs | 1. Sonicating the mixture of silver nanoparticles and sEVs (RT, 1 min). | Higher loading efficiency for cargoes with small size. |
|
73 |
| SiKeap1 |
|
74 | |||
| CA |
|
The introduction of albumin enhanced loading efficacy of curcumin by providing abundant binding-sites for curcumin inside sEVs. | 75 | ||
| Extrusion | Dapagliflozin |
|
High loading efficacy. |
|
76 |
| Paclitaxel |
|
77 | |||
| Clodronate |
|
78 | |||
| Electroporation | MiR-21-5p |
|
|
|
79 |
| MiR-31-5p |
|
80 | |||
| Didymin |
|
81 | |||
| Electroporation and CNP | COL1A1 mRNA |
|
High yield of sEVs with 103-fold increase in mRNA cargoes. | 82 | |
| Saponin treatment | β-glucuronidase |
|
Increasing loading efficiency of cargoes. |
|
83 |
| Co-incubation | VH298 | 1. Co-incubating the mixture of sEVs and VH298 at 37 °C for 1 h. |
|
Inferior loading efficacy and specificity. | 84 |
| MiR-21-5p |
|
Enhancing loading efficacy of miRNAs. | The grafting of YAYA may affect the bonding of miRNAs and mRNAs. | 85 | |
| pH gradients | MiRNAs, siRNAs and ssDNAs |
|
Enhancing loading efficacy of nucleic acid cargo without impairing cellular uptake and inducing toxicity response of sEVs. | May destroy membrane integrity of sEVs. | 86 |
| The combination of serial extrusion, ionic stress, and vesiculation | Myd88 peptides |
|
Obtaining high yield and purity of NVs. | The operation is cumbersome and the original therapeutic cargoes of sEVs is lost. | 87 |
- Abbreviations: Ag, NPs; silver, nanoparticles; CA, Curcumin; CNP, cellular nanoporation; iPS-ECs, induced pluripotent stem cells differentiated endothelial cells; L-CLD, clodronate loaded liposomes; Myd88, an anti-inflammatory peptide; NVs, nanovesicles; PC, polycarbonate; RT, room temperature; sEVs, small extracellular vesicles; siKeap1, siRNA-Keap1; TCEP, tris(2-carboxyethyl) phosphine; YARA, a cell-Penetrating Peptide.
4.1 Modifying parent cells
4.1.1 Genetic transfection
Since sEVs are released from their parent cells, it is reasonable to personalize sEVs by manipulating their parent cells. By genetic manipulation on parent cells, non-coding RNA sequences, cytosolic or targeting proteins can be displayed on the surface and/or enriched in the lumen of sEVs. Generally, the genetic transfection system consists of genetic cargoes and delivery systems. After construction, the genetic transfection system was co-cultured with parent cells to generate and select stable parent cell lines by adding puromycin. Next, the generated stable parent cell lines were cultured with a serum-free medium to collect engineered sEVs.88
Enriching cargoes in sEVs: The cargoes (i. e., miRNAs or proteins) inside sEVs can be modulated by genetically manipulating their parent cells.89, 90 MiR-31-3p-enriched sEVs were obtained by transfecting miR-31-3p lentiviral vector into HEK293 cells. The miR-31-3p-enriched sEVs promoted the angiogenesis, fibrogenesis and re-epithelization during diabetic wound healing by inhibiting factor-inhibiting HIF-1 (FIH) and epithelial membrane protein-1 (EMP-1).89 Similarly, the long non-coding RNA HOX transcript antisense RNA (HOTAIR, mediating angiogenic effects of endothelial cells) overexpressed sEVs were fabricated by transfecting BMMSCs with pCMV-HOTAIR plasmid.90 The HOTAIR-overexpressed sEVs facilitated diabetic wound healing by boosting angiogenesis.
Yang et al. launched a cutting-edge method named cellular nanoporation (CNP) to transfect mouse embryonic fibroblasts (MEFs), dendritic cells (DCs), MSCs and HEK293T cells on the array of nanochannels (diameter of 500 nm) with plasmid DNAs under a focal and transient electrical stimulus. With this approach, the yield and exosomal mRNA transcripts in collected engineered sEVs were both augmented markedly by 50 folds and 1000 folds, respectively.91 Subsequently, the CNP method was utilized to create sEVs enriched with COL1A1 mRNA, serving the purpose of collagen-replacement therapy for photoaged skin, and potentially offering insights into diabetic wound management.82 Additionally, a brand-new method called EXPLORs (sEVs for protein-loading by optically reversible protein-protein interactions) was developed to load soluble proteins inside sEVs with high efficacy.92, 93 As exemplified, super-repressor IκB (srIκB, the dominant active form of IκBα) was encapsuled massively into the lumens of sEVs to alleviate sepsis-related organ damage and inhibiting the secretion of proinflammatory cytokines. This approach involved the localization of CIBN-EGFP-CD9 and srIκB-mCherry-CRY2 on the surface and cytoplasm of HEK293T cells, respectively, achieved through co-transfection with corresponding expression plasmids. By applying a single pulse, the srIκB-mCherry-CRY2 protein was activated rapidly, allowing it to connect with CIBN-EGFP-CD9 at the plasma membrane during sEV biogenesis. The srIκB-mCherry-CRY2 protein was subsequently released into the lumen of sEVs upon the cessation of the single pulse radiation.94
Targeting and therapeutic function modification: Furthermore, the targeting proteins on the surface and therapeutic cargoes inside the lumen of sEVs can be engineered simultaneously. Recently, we designed a kind of engineered sEVs to load miR-146a and attach to silk fibroin efficiently by infecting placental MSCs (PMSCs) with two kinds of lentiviral particles simultaneously. The C1C2 domain of the sEV-specific protein MFGE8 (lactge8) was selected as the anchor site for sEVs, the silk fibroin binding peptide (SFBP) was modified at the N terminal, and the phage MS2 coating protein was modified at the C terminal. The SFBP screened by phage display technology increases the loading capacity of the sEVs onto the silk fibroin patch. The phage MS2-coated protein specifically binds to pac-modified RNA. Therefore, the loading efficiency of pac-pre-miR146a in sEVs were increased by approximately 10-fold compared with conventional methods.28
4.1.2 Co-culturing with cargoes
Culturing parent cells with drugs is usually employed to encapsule small hydrophobic drugs in the lumen of sEVs. These drugs were usually packaged in Golgi-derived vesicles in parent cells through endocytosis and were loaded in sEVs in a nonspecific and uncontrolled manner. The co-culturing was also combined with serial extrusion to generate engineered sEVs.94 The suspension of HEK293 cells in PBS was mixed with 1000 μg/mL of melatonin, followed by serial-extruding through PC membrane filters with diminishing pore sizes. Using this approach, melatonin was loaded into sEVs efficiently. The generated melatonin-enriched sEVs showed efficient anti-inflammatory effect to relieve atopic dermatitis.94
4.1.3 Changing culture parameters
Lipopolysaccharide (LPS) and cytokine stimuli: LPS and cytokines might be the most popular candidates to regulate the contents of sEVs. Ti et al. pretreated hucMSCs with LPS (100 ng/mL) for 2 days before the collection of sEVs. They found that the treatment of LPS changed the miRNA array of hucMSC-sEV cargoes, and enriched levels of let-7b, miR-1180, miR-183, miR-550b, and miR-133a, specifically. LPS pre-sEVs mediated the polarization of THP-1 to M2 phenotype through let-7b.95 Pretreating adipose MSCs (ADMSCs) with inflammatory cytokines, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) (40 ng/mL), enhanced the anti-inflammatory efficacy of ADMSC-derived EVs, shifting macrophages from M1 to M2 phenotype. The enhanced anti-inflammatory efficacy was attributed to the increased expression of miRNAs (miR-34a-5p, miR21, miR146a-5p).96
Hypoxia: Hypoxic preconditioning may be a popular tool to change therapeutic cargoes of sEVs. Umezu et al. revealed that hypoxia-resistant multiple myeloma produced sEVs with upregulated miR-135 for the first time.97 Murine BMMSCs were cultured under 1% O2 conditions for 72 h98 or in 0.5% O2 conditions for 24 h48 and ultracentrifuged to obtain Hypo-sEVs. The obtained hypo-sEVs were enriched with the antiapoptotic miR-125b-5p and miR-210, showing facilitated cardiac repair. Very recently, we cultured MSCs under hypoxic conditions (1% O2) at 37 °C for 48 h and collected the secreted sEVs.99 Specifically, miR-17-5p expression inside sEVs was elevated by hypoxic preconditioning and reduced the excessive neutrophil extracellular trap (NET) formation.
Chemical compounds: Some small molecular compounds were also employed to pretreat parent cells to get engineered sEVs with enhanced therapeutic effects.54, 100-103 Adding 1/128 dilution of the silicate ion solution to the culture medium of EPCs for 48 h, the contents including miR-126a-3p and angiogenic factors (VEGF, SDF-1, CXCR4 and eNOS) were significantly enriched inside engineered sEVs. The stimulation with silicate ions upregulated the expression of miR-126a-3p, which accounted for upregulating the expression of angiogenic factors in sEVs.54 In another study, Huang et al. reported that lncRNA H19 inside sEVs from atorvastatin-stimulated MSCs (1 μmol/L, 24 h) was upregulated by about 13 folds compared with those sEVs released from pristine MSCs.102 Besides, pretreating MSCs with pioglitazone was utilized to enhance the biological functions of secreted sEVs, including promoting migration, tube formation, and VEGF expression of HUVECs.100 The pretreatment of hBMMSCs with melatonin enhanced the anti-inflammatory effect of secreted sEVs.101 But the changed contents inside engineered sEVs were not studied by authors in depth. Furthermore, pretreating BMMSCs with iron oxide NPs (Fe3O4 NPs) under a static magnetic field (100 mT) resulted in a significant upregulation of miR-21-5p inside sEVs. These engineered sEVs were found to facilitate skin wound healing through the inhibition of SPRY2 and activation of the PI3K/AKT and ERK1/2 signaling pathways.104 These studies highlight the potential of chemical compounds to modulate the contents of engineered sEVs and enhance their therapeutic effects.
4.2 Functionalization of isolated sEVs
4.2.1 Modifying the membrane of isolated sEVs
Surface chemistry: Click chemistry, sulfhydryl-metal ion complex, and amidation reaction have been explored to decorate the surface of sEVs for the introduction of new functionalities or the enhancement of inherent therapeutic properties of sEVs. For example, Ruan et al. grafted stromal cell-derived factor 1 (SDF-1) on the surface of sEVs (derived from M2 microglia) via the copper-free click chemistry covalently, enhancing the migration and neuronal differentiation of neural stem cells (NSCs).67 Using DSPE-PEG-SH as the handle, Au3+ was captured on the surface of MSCs-sEVs to obtain MSCs-sEVs-SH-Au3+. Then, the mixture of ascorbic acid (AA) and NaBH4 at optimized ratio reduced Au3+ to gold NPs (AuNPs), while AA was the reducing and stabilizing agent on the surface of AuNPs. The decorated AA enhanced the relieving effect of MSCs-sEVs on dry eye diseases through synergetic scavenging reactive oxygen species (ROS) and reducing inflammation.68 The electrodynamic carboxylic group functionalized Pd-Pt nanosheets were patched on the surface of ginger-derived sEVs via an amidation reaction between the amino group and the carboxylic group. The patched Pd-Pt nanosheets endowed ginger-derived sEVs synergistic electrodynamic and photothermal effects towards anti-infective therapy, while ginger-derived sEVs allowed long circulation in vivo and the entrance of Pd-Pt nanosheet into bacteria.69 These approaches provide engineering principles of therapeutic sEVs for diabetic wound managements.
Hydrophobic interactions: A cholesterol conjugation strategy was explored to achieve the productive loading of siRNAs on the surface of sEVs through hydrophobic interactions. The siRNAs were pre-conjugated with the linkers of triethyl glycerol (TEG) or 2-aminobutyl-1-3-propanediol. Then, the cholesterol-conjugated siRNAs were co-incubated with sEVs at 37 °C for about 1 h. This approach allows ultrahigh loading efficacy of siRNAs inside sEVs (2500–3000 hsiRNAs per sEV), which is beneficial to silence specific genes in diabetic wounds.70 Another hydrophobic interaction-based strategy involves the covalent binding of a biomarker-specific peptide (P-selectin binding peptide, PBP) to DMPE-PEG5000-maleimide, which was then inserted into the phospholipid bilayer membranes of sEVs. This modification imparted imaging and therapeutic functions to the sEVs. The resulting PBP-decorated sEVs exhibited anti-inflammatory and angiogenesis-promoting effects, offering potential for the treatment of diabetic wounds.71
Electrostatic interactions: Electrostatic interaction was a characteristic non-covalent method to engineer sEVs. Fluorinated peptide dendrimers (FPG3) were grafted onto the surface of ADMSCs-sEVs through electrostatic interactions, creating sEVs@FPG3. This modification enhanced the intracellular delivery efficiency of sEVs by facilitating clathrin-mediated endocytosis. Moreover, sEVs@FPG3 exhibited improved tube-formation and migration abilities of HUVECs in vitro, which is essential for promoting angiogenesis in diabetic wounds.72
4.2.2 Modifying the contents of isolated sEVs
Ultrasonication: High-intensitive mechanical shear force under ultrasonication allows the membranes to reshape or the formation of transient pores on the surface of sEVs, opening the door for NPs, siRNAs and drugs from surrounding media to enter sEVs. Then, the engineered sEVs were incubated at 37 °C for 1 h to recover the opened membrane. For example, silver NPs (Ag NPs) were loaded into HucMSC-derived sEVs by sonicating the mixture for 1 min at room temperature. The obtained AgNP-loaded sEVs displayed anti-bacterial properties for the treatment of infective wounds.73 Besides, to relieve oxidative stress at diabetic wound sites, siRNA-Keap1 (siKeap1) functionalized milk-released sEVs were fabricated by ultrasonicating the mixed PBS solution of siRNA-Keap1 and sEVs. SiKeap1-enriched sEVs silenced Keap1 gene and increased the expression of antioxidant protein (HO-1) in vitro and accelerated diabetic wound healing by promoting collagen formation and neovascularization.74 Yerneni et al. loaded the anti-inflammatory drug (curcumin, CA) inside sEVs with the assistance of albumin under milder sonication. The pre-introduction of albumin provided abundant binding-sites of CA inside sEVs.75
Extrusion: Extrusion is another typical mechanical force to deform the membranes of sEVs. Extruding the mixture of sEVs and drugs to pass through a PC membrane with a pore size of 100–400 nm at a certain temperature and repeating for several times, the drugs were loaded into sEVs with high efficacy. For example, dapagliflozin was loaded into biomimetic NVs from induced pluripotent stem cell-derived endothelial cells (iPS-ECs) using repeated extrusion. This approach promoted angiogenesis at diabetic wounds by activating HIF-1α/VEGFA pathway.76 Zhang et al. packaged paclitaxel (PTX) into hucMSCs-sEVs by extruding the mixture through an Avanti lipid extruder with a 100 nm pore size repeatedly. The PTX-loaded hucMSC-sEVs enhanced neural regeneration and inhibited scar deposition.77 Besides, clodronate (CLD) loaded liposomes were mixed with fibroblast-derived sEVs and then they were subjected to vortex, sonicate and extrude through 400 and 200 nm PC membranes for 10 times. This hybrid system targeted lung fibrotic tissues efficiently, shedding light on inhibiting scar formation during diabetic wound healing.78
Electroporation: Electroporation is an efficient and popular tool to enhance the permeability of the phospholipid bilayer membrane of sEVs. Upon the stimulus of high-intensity electric field, transit pores appeared on the phospholipid bilayer membrane of sEVs, allowing the internalization of RNAs,79, 105 and drugs81 into sEVs. For example, miR-21-5p was loaded into ADMSCs-released sEVs by electroporation. The fabricated miR-21-5p-enriched sEVs promoted the proliferation and migration abilities of keratinocytes via Wnt/β-catenin signaling and facilitated diabetic wound healing by increasing re-epithelialization.79 Similarly, miR-31-5p mimics were also encapsuled in milk-derived sEVs through electroporation and this formulation accelerated angiogenesis at diabetic wound sites by improving endothelial cell functions.80
Additionally, the CNP method-based electroporation was employed to fabricate COL1A1 mRNA-enriched sEVs towards collagen-replacement therapy in photoaged skin, shedding light on optimizing collagen deposition at diabetic wounds.82 Hydrophobic drugs can be loaded into the lumen of sEVs using electroporation. Didymin-engineered sEVs were fabricated by mixing them in the cold electroporation buffer with the electroporation parameters of 0.35-s pulses 20 times of 0.7 kV.81 The obtained didymin-engineered sEVs provided a favorable immune-microenvironment for diabetic wound healing by switching the macrophage from M1 toward M2 phenotype. However, the method of electroporation may lead to siRNA precipitation and lower its biological activity.106 Despite its popularity in loading contents into sEVs, the optimization of electroporation parameters is needed urgently to ensure efficient loading and stabilities of cargoes.
Saponin treatment: Saponin could remove membrane-bound cholesterol on the surface of sEVs selectively, allowing the formation of transient holes/pores in the sEVs lipid bilayers. By virtue of surfactant-induced transient membrane destabilization, Fuhrmann et al. encapsulated ß-glucuronidase into hMSC-derived sEVs and fixed the engineered sEVs into a poly(vinyl alcohol) (PVA) hydrogel to achieve enzyme prodrug therapy (EPT) in a localized manner.83 The loaded ß-glucuronidase catalyzed glucuronide precursor into anti-inflammatory curcumin efficiently at local sites, providing inspirations for regulating inflammation during diabetic wound healing. However, rigorous purification steps are needed because this saponin-induced transient membrane destabilization may affect the stability of biomolecules.
Co-incubation: For the loading of hydrophobic drugs or nucleic acid drugs, co-incubation may be a more efficient and simpler solution. Recently, we loaded VH298 (a stabilizer of HIF-1α) inside ESC-sEVs with a loading efficacy of about 14% by mixing them at 37 °C for 1 h.84 The obtained VH298-loaded sEVs boosted angiogenesis both in vitro and in vivo. Interestingly, Hade et al. enhanced the loading efficacy of miRNAs inside sEVs by introducing a cell-penetrating peptide-equipped technology. Briefly, YARA (a cell-penetrating peptide) was conjugated with miR-21-5p covalently to obtain YARA-miR-21-5p. Then, YARA-miR-21-5p was loaded in sEVs by incubating them at room temperature. The loading efficacy of YARA-miR-21-5p was regulated by the co-incubating time and is 18.6 folds than control. The engineered sEVs boosted the cellular function of fibroblasts, which is crucial for diabetic wound healing.85
Other methods: Interestingly, Jeyaram et al. optimized the loading efficacy of nucleic acid cargoes inside sEVs through pH gradient method. In brief, the sEVs were dehydrated in 70% ethanol and rehydrated in the acidic citrate buffer. Then, the acid-rehydrated sEVs were dialyzed in the neutral HEPES-buffered saline, creating a pH gradient between the inner and outer membranes of sEVs. Next, these sEVs were incubated with nucleic acid cargoes, including miRNAs, siRNAs and single-stranded DNA (ssDNA), for 2 h at 22 °C according to the optimized loading parameters.86 Later, Park et al. obtained sEVs loading with Myd88 (an anti-inflammatory peptide) through a series of manipulations including serial extrusion of MSCs, ionic stress, and vesiculation. Briefly, extruded NVs were obtained by serial extruding MSCs through PC membrane with diminishing pore size. Then, these NVs were subjected in alkaline medium to obtain membrane sheets. Next, cholesterol anchor-premodifed Myd88 peptides were mixed with the obtained membrane sheets under mild sonication followed by iodixanol-based density cushion ultracentrifugation to obtain Myd88-loaded sEVs (sEVsMyd88).87
Noteworthily, it is necessary to combine various engineering methods skillfully to augment the therapeutic potential of engineered sEVs, just like building blocks. In further study, tailoring the targeting, therapeutic, and tracking properties of the same engineered sEVs need to be concerned subtly to ensure them to be delivered and exert their therapeutic efficacy maximumly in target tissues.
5 APPLICATIONS OF ENGINEERED sEVs IN DIABETIC WOUND MANAGEMENT
Reportedly, engineered sEVs have been involved in the healing processes of diabetic wounds modulating the function of skin repair cells including immune cells, vascular endothelial cells, keratinocytes, fibroblasts, myofibroblasts, and skin appendage-associated cells involved in the inflammatory phase, proliferative phase and remodeling phase (Figure 5).107 More importantly, sEVs can be easily customized to further improve their therapeutic and targeting efficiency.108 In this section, we summarized recent advances of engineered sEVs in the treatment of diabetic wounds.
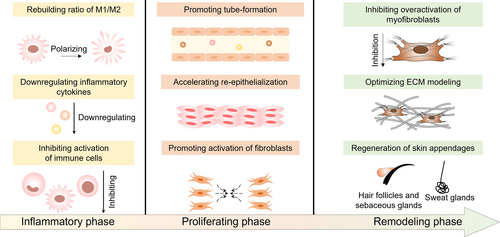
Engineered sEVs promoted diabetic wound healing process by modulating inflammatory phase, proliferating phase, and remodeling phase.
5.1 Modulating inflammatory phase
Excessive inflammation in diabetic wounds was recognized to impede the healing process.109 Recent researches illuminate that natural sEVs manifest the beneficial influence on the healing process of chronic wounds due to their ability to regulate macrophage polarization, reduce macrophage infiltration, and inhibit the production of ROS.110 However, there are certain functional constraints associated with these natural sEVs and the specific molecules instrumental in their function remain unidentified, hampering the progression of subsequent therapeutic strategies.111 In response to this, recent years have witnessed an escalation in the fabrication of engineered sEVs for the purpose of therapeutic interventions. These engineered sEVs with enhanced multi-functions by incorporating defined therapeutic cargoes demonstrate promising potential in the realm of chronic wound management.112
Rebuilding subtle ratio of M1/M2: In acute wounds, the appropriate inflammatory reaction helps clear invasions and necrotic tissues around the wound bed, creating a favorable environment for subsequent healing process. However, in diabetic wounds, excessive and uncontrolled inflammation delays the healing process.113 Many attempts have been made in recent years to develop sEVs-based strategies for the inflammatory regulation of diabetic wounds, and these efforts can be summarized mainly in three principles: restoring M1/M2 ratio, downregulating inflammatory cytokines and inhibiting immune cell activation.114
Macrophage polarization can be understood as a specific functional transformation under the trigger of certain conditions,115 and this transformation may play a central role in a variety of chronic inflammation-related diseases.116 M1 macrophages secrete pro-inflammatory factors such as interleukin-1β (IL-1β), IL-6 and IL-12, while M2 macrophages play a role in inhibiting excessive inflammation and promoting tissue repair by secreting anti-inflammatory cytokines such as IL-10 and Arg-1.117 The delayed transformation of macrophages hinder diabetic wound healing process. Therefore, macrophages are reasonably considered to be one of the therapeutic targets for alleviating excess inflammation.
By stimulating ADMSCs with pro-inflammatory factors, including IFN-γ/tumor necrosis factor-α (TNF-α), the anti-inflammatory functions of secreted ADMSCs-sEVs were enhanced.96 These engineered sEVs shifted macrophages from M1 to M2 phenotypes through shuttling miR-146 (accounted for regulating immune cell proliferation and inhibiting inflammatory responses via targeting IRAK-1 and TRAF6118, 119). In another study, the core-shell structured chimeric apoptotic bodies (cABs) were developed in which the membrane of ABs functioned as a bioconjugation/regulation shell and the mesoporous silica nanoparticles loaded with miRNA-21 or curcumin were the core.120 The developed cABs targeted macrophages in the wound region and shifted M1 to M2 phenotype to regulate inflammation on-demand through the concerto between the membrane of cABs and the intracellular release of miR-21 or curcumin. It is noteworthy that the natural chemotactic ability of T cells and the specific uptake of cABs by macrophages were exploited to achieve dual targeting, providing guidance for the subsequent development of more precise targeted therapy based on engineered sEVs.
Inhibiting excessive secretion of inflammatory cytokines: Reportedly, the engineered sEVs were able to inhibit the secretion of inflammatory cytokines. Curcumin, a typical antioxidant and anti-inflammatory drug,121 was limited by its low solubility and in vivo stability during practical applications.122 Phospholipid bilayer membranes of sEVs may help overcome these drawbacks. In one study, sEVs were sequentially loaded with albumin and curcumin by mild sonication to improve the solubility, stability and loading efficacy of curcumin in vivo,75 followed by being encapsulated in the soluble microneedle array (MNA). The curcumin-loaded sEVs inhibited and reduced LPS- and imiquimod-induced inflammatory cytokine release by reversing the expression of the inflammatory transcription factor NF-κB in rat and mouse models, significantly decreasing the infiltration of inflammatory cells at wound site.
In addition to small molecule drugs, a variety of miRNA cargoes inside sEVs are involved in the expression regulation of inflammatory factors.123 Recently, we successfully engineered sEVs by constructing fusion proteins SFBP-Gluc-MS2 (SGM) and pac-miR146a-pac, in which the exosomal membrane protein Lactadherin was fused to RNA binding protein and SFBP.28 This engineering strategy significantly improved the therapeutic efficacy of sEVs. The expression of inflammatory cytokines IL-1β, IL-6 and TNF-α in HaCaT cells was significantly downregulated by SGM-miR146a-sEVs through silencing the expression of IRAK1 (one of the upstream regulators of the NF-κB signaling pathway).
Inhibiting overproduction of immune cells: During the inflammatory phase, dysfunctions or over-activation of innate or adaptive immune cells in diabetic wounds also impaired the healing process.124 Recently, we noticed that the hyper-activated neutrophils in diabetic wounds led to excessive formation of NETs.99 We observed that sEVs obtained from hypoxia-treated MSCs blocked the formation of NETs by delivering enriched miR-17-5p (targeting the TLR4/ROS/MAPK pathway) to promote diabetic wound healing.99 In another study, HLA-A2, co-stimulatory CD80 and/or co-inhibitory programmed death ligand 1 (PD-L1) were decorated on the surfaces of sEVs.125 The conjunction of surface biomolecules confers the ability of the sEVs to regulate T cells in Type 1 diabetes. This work broadens the scope of target cells for engineered sEVs and provides inspiration for engineered sEVs targeting other immune cells at wound sites.
5.2 Regulating proliferating phase
Several crucial cellular behaviors, including re-epithelialization, angiogenesis, and fibroblast activation, occur during the proliferative phase.126, 127 Recent studies have demonstrated that natural sEVs may exert therapeutic effects by enhancing the proliferation and migration,23, 128 and mitigating oxidative stress-induced senescence of HUVECs,129 or inhibiting the apoptosis and recovering cellular function of HaCaTs.130 However, the heterogeneity of natural sEVs produced by the same cell under different physiological conditions poses challenges for both research and clinical applications.131 Consequently, treatment strategies utilizing engineered sEVs may partially overcome the limitations associated with natural sEVs.132
Promoting angiogenesis: Angiogenesis, accounting for the transportation of nutrients and growth factors to the wound bed, is usually difficult during the proliferative phase of diabetic wounds.4 Therefore, one of the key targets for engineered sEVs-based therapeutic strategies is to promote angiogenesis.129 Recently, we made an attempt to load VH298, which could activate HIF-1α-mediated gene expression including VEGF, into the lumen of sEVs by co-incubating them at 37 °C for 1 h (Figure 6A,B).84 VH298 engineered sEVs promoted the proliferation (Figure 6C) and tube formation (Figure 6D) of HUVECs. Further encapsulating VH298-loaded sEVs into gelatin methacryloyl hydrogel boosted blood perfusion and neovascularization in vivo at D12 mediated by HIF-1α-enhanced angiogenesis (Figure 6E).84 In another study, a core-shell MNA was developed by encapsulating sEVs from iron nanoparticles (Fe NPs) pretreated MSCs within the inner core and polydopamine (PDA) NPs were employed as the outer shell.133 Reportedly, treating BM-MSCs with Fe NPs stimulates the secretion of sEVs (Fe-MSC-EVs) with more therapeutic cytokines such as HGF, VEGF, angiopoietin-1 (Ang-1) and fibroblast growth factor-2 (FGF-2) to promote angiogenesis.133 The combination of antioxidant and anti-inflammatory properties of PDA NPs and pro-angiogenesis of Fe-MSC-sEVs showed excellent effect on diabetic wound healing.133 Interestingly, researchers found that sEVs derived from neonatal serum-educated MSCs promoted endothelial cells to form tubes and blood vessels via regulating AKT/eNOS pathway.134 Despite that researchers have identified many angiogenesis-promoting targets of engineered sEVs,135 metrics to assess the function and structure of neovessels and investigate targets related to neovessels maturation need more attention and to be established.
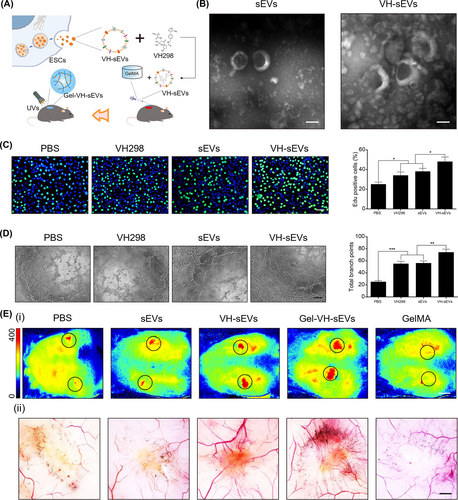
Application of VH-sEVs in accelerating angiogenesis. Reproduced with permission.84 Copyright 2022, Elsevier. (A) Schematic showing the fabrication and in vivo application of VH298-loaded sEVs. (B) Morphologies of sEVs and VH-sEVs. Scale bar = 100 nm. (C) EdU assay and analysis showing the proliferation of HUVECs in different treatment groups. (D) Tube formation and statistical analysis of HUVECs with different treatments. Scale bar = 100 μm. (E) Blood perfusion (i), scale bar = 1 cm, and neovascularization (ii), scale bar = 3 mm, in wound with different treatments.
Accelerating re-epithelialization and activation of fibroblast: Re-epithelialization occurs during the proliferative phase of wound healing, mainly involving fibroblasts and epithelial cells at the edge of the wounds.136, 137 However, the complex pathophysiological environment in diabetic wounds impairs this process. A variety of engineered sEVs with altered compositions have been developed to regulate cell behaviors during the proliferative phase. Huang et al. reported miR-31-5p-loaded sEVs promoted proliferation and migration of fibroblasts and epidermal cells by regulating HIF and EMP-1 signaling pathways, hence accelerating re-epithelialization at diabetic wounds.89 Similarly, Lv et al. enveloped miR-21 into sEVs by electroporation and employed them to boost fibroblast differentiation and wound epithelialization, offering an option for future drugs and cell-free therapies for the treatment of diabetic wounds.79 Another way to modify sEVs is to pre-treat the parent cells.138 Wang et al. showed significant differences between the contents of sEVs harvested after hypoxic pretreatment (HypADSC-sEVs) and the contents of sEVs from ADMSCs without hypoxic treatment. Among these differentially expressed genes, upregulation of miR-21, miR-126, miR-31 with downregulation of miR-99b and miR-146a may be associated with diabetic wound healing. In addition, HypADSC-sEVs could accelerate re-epithelialization through the PI3K/AKT pathway.139
Recently, the combination of novel biomaterials with sEVs caught the eye of researchers. Wang et al. enveloped sEVs into pH-responsive antibacterial hydrogel, achieving re-epithelization, proper collagen deposition and complete skin regeneration.140 Jiang et al. developed matrix metalloproteinase (MMP)-responsive smart hydrogel to encapsulate ADSC-sEVs to reduce undue oxidative stress and to improve fibroblast proliferation, realizing faster wound healing.141 Thus, the application of functional biomaterials allowed sustainable release of sEVs, making a preferable sEV-carrier for synergistic efficacy.
Re-epithelialization and fibroblast activation are the most critical cellular behaviors during the proliferative phase. However, the choice of target cells should not be limited to epidermal cells and fibroblasts, but focused on the whole process of promoting diabetic wound healing. Future strategies to engineer sEVs should focus on broadening the range of therapeutic molecules and new therapeutic targets.
5.3 Optimizing remodeling phase
The remodeling phase usually occurs at the final healing process, during which a key cellular behavior is the secretion and degradation of ECM to increase the tissue strength of the wound while reducing the thickness of the scar.142 However, this key behavior needs sophisticated regulation because poor deposition of ECM may lead to reduced strength of the trauma, while excessive activation of myofibroblasts may lead to the proliferation of scar tissue. In recent years, there has been growing interest in the potential of natural sEVs derived from adipose-derived mesenchymal stem cells for regulating scar formation. During the early stage, these sEVs accounted for regulating the subtle ratio of collagen I (Col I) to collagen III (Col III). In the later stage, they contributed to reducing scar formation by inhibiting excessive collagen production.143, 144 However, when it comes to clinical applications, high dosages of natural sEVs are often required for the effective delivery of bioactive ingredients to receptor cells, resulting in increased time and economic costs.144 To address these challenges, various engineering methods have been developed to enhance the enrichment of specific molecules within sEVs for scar treatment.
Inhibiting overactivation of myofibroblasts: Aggregation of myofibroblasts is one reason for excessive scar formation. To fix this, Fang et al. found that hucMSCs-sEVs reduced scar formation by limiting the accumulation of myofibroblasts by a group of miRNAs (miR-21, -23a, −125b and −145) mediated TGF-β/SMAD2 pathway inhibition in a skin defect mouse model.145 Significantly, these identified therapeutical miRNAs provide novel ideas for the subsequent design of engineered sEVs. Likewise, Jiang et al. obtained engineered sEVs loaded with TSG-6 protein, by lentiviral infection of MSCs for overexpression of TSG-6. The results showed that sEVs loaded with TSG-6 significantly reduced the levels of α-SMA, a cellular marker of myofibroblasts.146
Optimizing ECM modeling: Indeed, factors affecting scar tissue formation are not only limited to the activity of fibroblasts during the remodeling phase. Actually, the whole process of wound healing has the potential to influence the production of scar tissue.147 However, it is difficult for conventional treatments to promote wound healing while inhibiting the formation of scar tissue. To overcome this, the release of the engineered sEVs with different functions at different times can be achieved by combining engineered sEVs with biosynthetic materials. Shen et al. identified Col A1 as a direct target gene of miR-29b-3p based on a bioinformatics algorithm. Then, they designed a double-layer hydrogel dressing in which the miR-29b-3p-enriched BMMSC-sEVs were encapsulated at the upper layer and the BMMSC-sEVs were loaded at the lower layer (Figure 7A). The sustained release of the miR-29b-3p-enriched BMMSC-sEVs inhibited excessive vascular proliferation and collagen deposition during the late proliferation phase (Figure 7B,C), resulting in scarless wound healing (Figure 7D,E).148
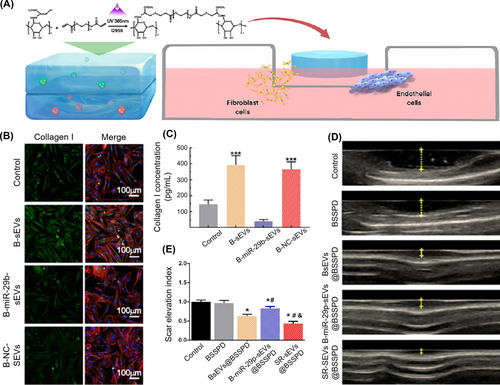
Application of sEVs released from bilayered hydrogels for scarless wound healing. Reproduced with permission.148 Copyright 2021, American Chemical Society. (A) Design of bilayered hydrogels loaded with BMMSC-sEVs at lower layer and BMMSC-miR-29b-sEVs at upper layer. (B and C) Immunofluorescence staining images and analysis demonstrating the secretion of Col I from HFF-1 cells. (D and E) Ultrasonography of wounds and quantitative analysis of the scar elevation index.
Most studies have shown that collagen synthesis and degradation are crucial for scar formation. Theoretically, excess Col III during the remodeling phase was degraded and replaced by mature Col I.149 However, in diabetic wounds, the ratio of Col I/Col III is difficult to achieve an ideal balance during the remodeling phase, and myofibroblasts tend to be over-activated, both of which led to a decrease in the quality of wound healing.150 Furthermore, low-intensity wounds, or the scarring that results from healing wounds, can be painful for the patient. The strategies based on sEVs offer the opportunity to get closer to perfect healing. However, the therapeutic effects of natural sEVs have varied significantly across studies.130, 151 Therefore, the development of more therapeutic strategies with more rational engineered sEVs for therapeutic targets in the remodeling phase is eagerly awaited.
Accelerating regeneration of skin appendages: Skin appendages include hair follicles, sweat glands and sebaceous glands. As important parts of the complete skin structure, these appendages are involved in thermoregulation, sebum production, vascularization, skin immune response and wound healing.152 However, the outcome of diabetic wounds is often associated with significant loss of skin appendages,140 leading to disturbed temperature perception, impaired skin barrier integrity, and loss of self-confidence.153 Up to now, sEVs-based strategies to modulate the microenvironment for the regeneration of skin appendages have proven more feasible.154-156
Activation of hair follicle stem cells (HFSCs) can promote hair follicle regeneration.157 Based on this phenomenon, Yang et al. developed a drug delivery system consisting of keratin MNA for the continuous delivery of MSCs-sEVs and small molecule drug UK5099 as an HFSC activator to the hair follicle microenvironment to induce upregulation of hair cycle activation-related protein expression.154 Clearly, this study is an exemplary strategy for combining sEVs with biomaterials. Dermal papilla cells are one of the key participants in the hair follicle cycle and hair follicle regeneration.153 Cao et al. prepared sEVs from neural progenitor cells by means of sequential extrusion and found that the obtained sEVs promoted DPC proliferation and HF growth.155 The possible mechanism was associated with the miR-100-mediated activation of the Wnt/β-catenin signaling pathway in sEVs.155
Similarly, sweat glands are one of the important skin appendages and are essential for the metabolic and sensory functions of the skin.158 Chen et al. developed the TGF-β-enriched sEVs to promote functional wound healing and sweat gland regeneration (Figure 8A).156 The TGF-β-enriched sEVs derived from MSCs could promote the migration of epidermal keratinocytes, phenotypic plasticity in migrating keratinocytes (Figure 8B), thus quickly closing wounds and promoting the regeneration of functional sweat glands (Figure 8C,D). Unfortunately, the authors did not claim the detailed mechanism.

The effect of TGF-β1-enriched sEVs in accelerating re-epithelization inducing regeneration of sweat glands. Reproduced with permission.156 Copyright 2022, Elsevier. (A) Schematic for preparing TGF-β1-enriched sEVs. (B) Immunofluorescence staining and analysis demonstrating Sox9+ keratinocytes. (C) Starch-iodine tests displaying the regeneration of sweat glands in the paw skin of thermal-injured mice at D16. (D) H&E staining and corresponding analysis showing the emerged glandular structures in the dermis of TGF-β1-sEV-treated mice.
Overall, the regeneration of skin appendages remains one of the toughest challenges during diabetic wound healing in recent years.152 Excitingly, a considerable number of studies have reported promising therapeutic strategies.153, 159 Among these, sEVs-based strategies, as the research hotspots, have shown superiority over cellular therapies and biological scaffolds.160, 161 In addition to MSC-sEVs, an increasing number of sEVs from other cell sources (i. e., keratinocytes and platelets) are gradually being revealed to promote the regeneration of skin appendages.162, 163 However, the development of skin appendages164 and the microenvironment of diabetic wounds are currently poorly understood.165 The therapeutic molecules developed are limited and the potential therapeutic targets are unclear.166 In the future, the design of engineered sEVs should be optimized to improve their efficiency, specificity and safety in regenerating skin appendages.
6 PERSPECTIVES AND OUTLOOK
Since sEVs were first reported in 1946, they have provided a rich palette for designing next-generation theranostic platforms. As important mediators for intercellular communication, sEVs can be secreted by various donor cells and regulate the functions of proximal or distal target cells. Recently, sEVs have been recognized as promising candidates for the treatment of chronic wounds due to their therapeutic potential and drug-loading capacity. Compared with MSC-based therapy, sEV-based therapy shows several advantages. Firstly, the tumorigenicity of sEVs is lower than that of MSCs. MSCs after transplantation were reported to undergo chromosomal abnormalities at early passages and form malignant tumors in heart.167 Secondly, the immune rejection of sEVs is lower than that of MSCs (especially the allogeneic MSCs, allo-MSCs).168 Thirdly, sEVs can cross multi-biological barriers, especially the blood-brain barrier, suitable for the treatment of nervous system disease.88 Fourth, the dose of sEVs is more controllable, which is beneficial for establishing the standardized operating procedures.
However, low yields, complex composition as well as inferior therapeutic and targeting effects have restricted their clinical application. Based on this, various methods have been explored to enhance the yields of sEVs and improve their targeting or therapeutic function as mentioned above. Nevertheless, several issues need to be addressed before the clinical translation of sEVs. Firstly, despite tremendous advances in regulating sEVs' production and cargo loading, the existing strategies carry the risk of altering parent cell phenotype, sEVs' function, and/or generating toxic or immunogenic response in vivo. Secondly, although there have been some pioneering attempts to achieve both mass-production and functionalization of sEVs,63 the processes of mass-production and functionalization are generally independent in most cases. How to achieve the mass-production of functionalized sEVs and to achieve an ingenious balance between the mass production and functionalization of sEVs? Thirdly, the critical size of cargoes loaded in sEVs should be considered when we choose the engineering methods. For example, engineering parent cells may be the preferential strategy to load large-sized cargoes such as circular RNAs (circRNAs) into secreted sEVs because the modification strategies after sEV secretion hardly create a big hole on the phospholipid bilayer membrane of sEVs. Fourth, in the context of diabetic wounds, our current understanding of their pathogenesis is still limited. The local microenvironment of these wounds is known to be harsh and complex, which poses a potential risk of compromising the therapeutic efficacy of sEVs. Fifth, standardized models of diabatic wounds need to be established for comprehensive evaluation of sEVs to avoid inconsistency between human diabetic wounds and diabetic rodent wounds before human trials. Finally, the clinical implementation of sEV-based treatment encounters numerous obstacles, including ensuring safety, verifying efficacy, adhering to production protocols, complying with regulatory guidelines, and managing patient recruitment, registration, and retention. Convincedly, the sEV-based therapy will be the encouraging choice for the management of diabetic wounds once the yields and engineering bottlenecks were broken.
ACKNOWLEDGMENTS
X. Liu, Q. Wei and Z. Sun contributed equally to this work. This study was supported by the National Nature Science Foundation of China (22205260, 82172211, 92268206, 81830064, 21972155 and 22002177), National Key Research and Development Programs of China (2022YFA1104303), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5–059), the Military Medical Research Projects (145AKJ260015000X; 2022-JCJQ-ZB-09600), Military Medical Science and Technology Youth Training Program (21QNPY128), Youth Innovation Promotion Association of CAS (No. 2022027) and International Partnership Program of Chinese Academy of Sciences (1A1111KYSB20200010).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Biographies

Xi Liu is an assistant researcher at Medical Innovation Research Division of Chinese PLA General Hospital (Beijing, China). She received her Ph.D. degree (2021) under the supervision of Prof. Shutao Wang at the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences (TIPC, CAS). Her research interests include engineered sEVs, bioinspired adhesives, wound healing, multistage immune regulation, and electrospinning.

Qian Wei is a postdoctor at Research Center for Tissue Repair and Regeneration Affiliated to the Medical Innovation Research Division and a doctor in the Department of Burns and Plastic surgery affiliated to the 4th Medical Center of Chinese PLA General Hospital. She received her Ph.D. degree (2021) under the supervision of Prof. Xiaobing Fu at Chinese PLA Medical school. Her research interests include engineered sEVs, mesenchymal stem cells, wound healing, and angiogenesis.

Zijie Sun received his B.S. degree from the Chinese PLA Air Force Military Medical University in 2022. He is currently a Ph.D. candidate at the PLA Medical School under the supervision of Prof. Xiaobing Fu. His research focuses on engineered sEVs, sweat gland regeneration and tissue-engineered skin.

Lianxin Shi is an associate professor at TIPC, CAS. He received his Ph.D. degree in 2019 from TIPC, CAS under the supervision of Prof. Shutao Wang. Then, he worked at TIPC, CAS as an assistant professor (2019–2021). He was appointed as an associate professor in 2021. He focuses on developing adhesives suitable for extreme conditions and anti-adhesive self-pumping dressings.

Cuiping Zhang obtained her Ph.D. degree in Pathology and Pathophysiology from the Academy of Military Medical Sciences in 2006. Currently, she is a full Professor at the Medical Innovation Research Division of Chinese PLA General Hospital (Beijing, China). Her current research focuses on tissue repair and regeneration.

Shutao Wang is a full professor at TIPC, CAS. He obtained his Ph.D. degree in 2007 at the Institute of Chemistry, CAS (IC, CAS). Subsequently, he worked as a postdoctoral researcher in the Department of Molecular and Medical Pharmacology and California NanoSystem Institute at the University of California in Los Angeles. He was appointed as a full Professor of Chemistry in 2010 at IC, CAS. His scientific interests focus on the design and synthesis of bio-inspired interfacial materials with special adhesion and their applications at the nano-biointerface.

Xiaobing Fu is a full professor at Chinese PLA General Hospital. He received his Ph.D. degree in 1993 at University of Madrid, Spain. He was appointed as a full Professor in 1995 at PLA 304th Hospital. He was elected as an academician of the Chinese Academy of Engineering in 2009. His scientific interests focus on tissue repair and functional skin regeneration in war trauma and post-trauma, especially on the regeneration of skin appendages, including sweat glands, sebaceous glands as well as hair follicles.




