Comparison of different EM methods for observation of lipidic structures in the cell nucleus
Abstract
Permanent progress in electron microscopy (EM) techniques enables us to reveal the finest ultrastructural details of the cell nucleus. Our recent work is focused on the previously unrecognized nucleoplasmic structures composed of phosphatidylinositol 4,5-bisphosphate, so called PIP2 islets. The super-resolution light microscopy enabled us to localize PIP2 islets inside the cell nucleus and reveal their colocalization with several components of Pol II transcription machinery and chromatin. However, only EM provides sufficient resolution to reveal the PIP2 islets ultrastructure and composition via immunostaining.
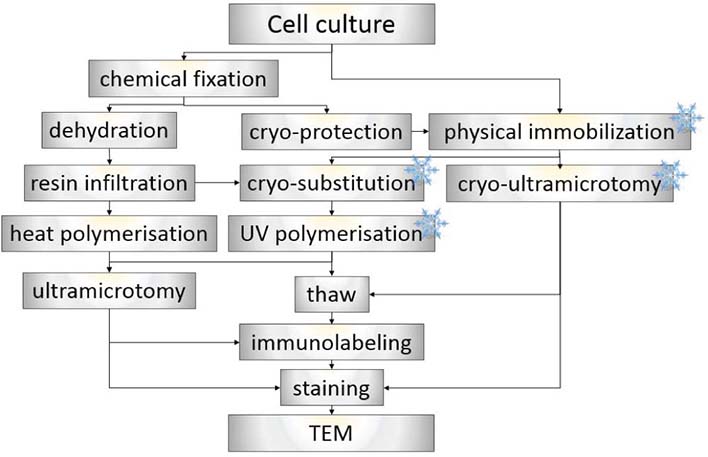
The usage of appropriate method for EM sample preparation is a crucial step in the whole procedure as PIP2 islets are lipidic structures which are predisposed to extraction or translocations. Here we compare different EM sample preparation techniques which we have optimized for the best ultrastructural preservation and antigenicity retention of PIP2 islets. We have analyzed several chemical fixation and cryoimmobilization approaches followed by various embedding media including both acrylic and epoxy resins. Moreover, different immunolabeling techniques were used such as pre-embedding, on-section labeling, and Tokuyasu method. With all these approaches we were able to observe roundish PIP2 islets (40-100 nm) recognized by anti-PIP2 antibodies. Preliminary results demonstrate that PIP2 islets are stable nuclear structures resistant to aldehyde fixation and extraction during preparation and cutting procedures. Based on the upcoming statistical analysis, the best approach for immunological studies of PIP2 islets will be selected.