Aminocyclopentadienyl Ruthenium Chloride: Catalytic Racemization and Dynamic Kinetic Resolution of Alcohols at Ambient Temperature†
Jun Ho Choi
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorYu Hwan Kim
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorSe Hyun Nam
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorSeung Tae Shin
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorMahn-Joo Kim Prof.
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorJaiwook Park Prof.
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorJun Ho Choi
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorYu Hwan Kim
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorSe Hyun Nam
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorSeung Tae Shin
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorMahn-Joo Kim Prof.
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorJaiwook Park Prof.
National Research Laboratory of Chirotechnology Department of Chemistry Division of Molecular and Life Sciences Pohang University of Science and Technology (POSTECH) San 31 Hyoja Dong, Pohang 790-784 (Korea) Fax: (+82) 54-279-3399
Search for more papers by this authorWe are grateful for the financial support from MOST through the NRL program, KOSEF through the Center for Integrated Molecular System, and the Korean Ministry of Education through the BK21 project for our graduate program.
Graphical Abstract
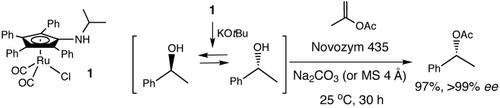
Ruthenium–enzyme tandem catalysis: The novel racemization catalyst 1 improves the dynamic kinetic resolution (DKR) of secondary alcohols dramatically. The DKR proceeds at room temperature with isopropenyl acetate as an acyl donor. In addition, the DKR is faster even with much less lipase than in previous DKRs.
References
- 1 For recent reviews, see:
- 1a R. Noyori, M. Tokunaga, K. Kitamura, Bull. Chem. Soc. Jpn. 1995, 68, 36;
- 1b R. S. Ward, Tetrahedron: Asymmetry 1995, 6, 1475;
- 1c S. Caddick, K. Jenkins, Chem. Soc. Rev. 1996, 447;
- 1d
R. Stürmer, Angew. Chem. 1997, 109, 1221;
10.1002/ange.19971091107 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1173;
- 1e U. T. Strauss, U. Felfer, K. Faber, Tetrahedron: Asymmetry 1999, 10, 107;
- 1f F. F. Huerta, A. B. E. Minidis, J.-E. Bäckvall, Chem. Soc. Rev. 2001, 30, 321.
- 2
- 2a P. M. Dinh, J. A. Howarth, A. R. Hudnott, J. M. J. Williams, W. Harris, Tetrahedron Lett. 1996, 37, 7623;
- 2b
A. L. E. Larsson, B. A. Persson, J.-E. Bäckvall, Angew. Chem. 1997, 109, 1256;
10.1002/ange.19971091123 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1211;
- 2c B. A. Persson, A. L. E. Larsson, M. L. Ray, J.-E. Bäckvall, J. Am. Chem. Soc. 1999, 121, 1645;
- 2d B. A. Persson, F. F. Huerta, J.-E. Bäckvall, J. Org. Chem. 1999, 64, 5237;
- 2e F. F. Huerta, Y. R. S. Laxmi, J.-E. Bäckvall, Org. Lett. 2000, 2, 1037;
- 2f F. F. Huerta, J.-E. Bäckvall, Org. Lett. 2001, 3, 1209;
- 2g J. H. Koh, H. M. Jeoung, M.-J. Kim, J. Park, Tetrahedron Lett. 1999, 40, 6281;
- 2h H. M. Jeong, J. H. Koh, M.-J. Kim, J. Park, Org. Lett. 2000, 2, 2487;
- 2i H. M. Jeong, J. H. Koh, M.-J. Kim, J. Park, Org. Lett. 2000, 2, 409;
- 2j M.-J. Kim, D. Lee, E. A. Huh, H. M. Jeong, J. H. Koh, J. Park, Org. Lett. 2000, 2, 2377;
- 2k M.-J. Kim, Y. K. Choi, M. Y. Choi, M. J. Kim, J. Park, J. Org. Chem. 2001, 66, 4736.
- 3 In reference [2g], we have reported an exceptional case that does not require hydrogen mediators. However, oxygen, a base, and high reaction temperature were necessary to generate catalytic species.
- 4 In references [2h] and [2i], we have described the use of alkenyl acetates as substrates and acyl donors in the presence of proper hydrogen donors.
- 5 For a review about racemization, see: E. J. Ebbers, G. J. A. Ariaans, J. P. M. Houbiers, A. Braggink, B. Zwanenburg, Tetrahedron 1997, 53, 9417.
- 6 We have found a catalyst system for the racemization of secondary alcohols at room temperature without the aid of hydrogen mediators. However, the catalyst system requires strong bases, and is not compatible with enzymatic acylation: J. H. Koh, H. M. Jeong, J. Park, Tetrahedron Lett. 1998, 39, 5544.
- 7 Crystal data for 1: C34H28ClNO2Ru. Mr=619.09 g mol−1. Orange crystal: size 0.50×0.20×0.20 mm3, orthorhombic, a=20.1560(13), b=13.2405(9), c=21.1819(14) Å, α=90, β=90, γ=90°, space group Pbca, V=5652.9(6) Å3, Z=8, T=223(2) K, ρcalcd=1.455 g cm−3, absorption coefficient=0.681 mm−1. Siemens SMART diffractometer, λ=0.71073 Å, scan mode-ω (ω-scan width: 1.92 to 23.33°). 22 982 reflections were measured, giving 4077 unique data with I>2σ(I). R=0.0252, Rw=0.0592, GOF=1.004. CCDC-179224 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 8
For examples of activating catalyst precursors by treatment with proper bases, see: K.-J. Haack, S. Hashiguchi, A. Fujii, T. Ikariya, R. Noyori, Angew. Chem. 1997, 109, 297;
10.1002/ange.19971090333 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 285, and references therein.
- 9 The separation of the product from unreacted p-chlorophenyl acetate is difficult in some cases.[2c]
- 10 When 5 mol % of acetic acid was added to the mixture given in entry 1 in Table 1, the optical purity of 1-phenylethanol changed only to 69.0 % ee in 30 min. However, the racemization was completed in 5 h by the subsequent addition of sodium carbonate (1 equiv).
- 11 A related complex, [{2,5-Ph2-3,4-tol2(η5-C4COH)}Ru(CO)2(O2CCF3)], is formed in the reaction of [{2,5-Ph2-3,4-tol2(η4-C4CO)Ru(CO)2}2] and trifluoroacetic acid: C. P. Casey, S. W. Singer, D. R. Powell, R. K. Hayashi, M. Kavana, J. Am. Chem. Soc. 2001, 123, 1090.
- 12 The imine was prepared in 90 % yield according to the procedure described by W. Dai, R. Strinivasan, J. A. Katzenellenbogen, J. Org. Chem. 1989, 54, 2204. Physical properties of the imine: m.p.: 223 °C; 1H NMR (CDCl3): δ=7.25–6.75 (m, 20 H), 4.08–4.00 (m, 1 H), 1.04 ppm (d, J=3 Hz, 6 H); 13C NMR (CDCl3): δ=165.8, 137.6, 131.9, 130.2, 129.8, 128.2, 127.8, 127.4, 127.2, 127.1, 126.5, 52.3, 24.6 ppm; MS (FAB, m/z): 425.27 (M+); elemental analysis (%) calcd for C32H27N: C 90.31, H 6.39, N 3.29; found: C 90.26, H 6.62, N 3.14.



41:13<>1.0.co;2-h.cover.gif)
