Synthesis of Biologically Potent α1→2-Linked Disaccharide Derivatives via Regioselective One-Pot Protection–Glycosylation†
Cheng-Chung Wang
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorJinq-Chyi Lee
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorShun-Yuan Luo
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorHsin-Fang Fan
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorChin-Ling Pai
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorWei-Chieh Yang
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorLung-Dai Lu
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorShang-Cheng Hung Dr.
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorCheng-Chung Wang
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorJinq-Chyi Lee
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorShun-Yuan Luo
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorHsin-Fang Fan
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorChin-Ling Pai
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorWei-Chieh Yang
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorLung-Dai Lu
Department of Chemistry, National Tsing Hua University Hsinchu 300 (Taiwan)
Search for more papers by this authorShang-Cheng Hung Dr.
Institute of Chemistry, Academia Sinica Taipei 115 (Taiwan) Fax: (+886) 2-2783-1237
Search for more papers by this authorWe thank Professor Sunney I. Chan for his helpful discussions. This work was supported by the National Science Council (NSC 90-2323-B-001-008).
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18593_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a
F. Burkhart, Z. Zhang, S. Wacowich-Sgarbi, C.-H. Wong, Angew. Chem. 2001, 113, 1317–1319;
10.1002/1521-3757(20010401)113:7<1314::AID-ANGE1314>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1274–1277;10.1002/1521-3773(20010401)40:7<1274::AID-ANIE1274>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 1b
T. Zhu, G.-J. Boons, Angew. Chem. 1999, 111, 3704–3707;
10.1002/(SICI)1521-3757(19991203)111:23<3704::AID-ANGE3704>3.0.CO;2-V Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3495–3497;10.1002/(SICI)1521-3773(19991203)38:23<3495::AID-ANIE3495>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 1c J. M. Lassaletta, R. R. Schmidt, Liebigs. Ann. 1996, 1417–1427;
- 1d M. T. Bilodeau, T. K. Park, S. Hu, J. T. Randolf, S. J. Danishefsky, P. O. Livingston, S. Zhang, J. Am. Chem. Soc. 1995, 117, 7840–7841.
- 2
- 2a T. Feizi, Nature 1985, 314, 53–57;
- 2b B. Wegmann, R. R. Schmidt, Carbohydr. Res. 1984, 184, 254–261;
- 2c J.-C. Jacquinet, P. Sinaÿ, Tetrahedron 1976, 32, 1693–1697.
- 3
- 3a C. Kunz, S. Rudloff, W. Baier, N. Klein, S. Strobel, Annu. Rev. Nutr. 2000, 20, 699–722;
- 3b M. T. Cancilla, S. G. Penn, J. A. Carroll, C. B. Lebrilla, J. Am. Chem. Soc. 1996, 118, 6736–6745.
- 4 T. Biswas, A. K. Mukherjee, Carbohydr. Res. 1978, 63, 173–181.
- 5 M. B. Perry, D. R. Bundle, V. Daoust, D. J. Carlo, Mol. Immunol. 1982, 19, 235–246.
- 6
- 6a
K. C. Nicolaou, C. N. C. Boddy, S. Bräse, N. Winssinger, Angew. Chem. 1999, 111, 2230–2287;
10.1002/(SICI)1521-3757(19990802)111:15<2230::AID-ANGE2230>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2096–2152;10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 6b
D. H. Williams, B. Bardsley, Angew. Chem. 1999, 111, 1264–1286;
Angew. Chem. Int. Ed. 1999, 38, 1172–1193;
10.1002/(SICI)1521-3773(19990503)38:9<1172::AID-ANIE1172>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 6c C. Thompson, M. Ge, D. Kahne, J. Am. Chem. Soc. 1999, 121, 1237–1244.
- 7 C. A. A. van Boeckel, P. Westerduin, J. H. van Boom, Carbohydr. Res. 1984, 133, 219–234.
- 8 S. K. Heatherington, J. Baddiley, Biochem. J. 1968, 107, 491–496.
- 9 For excellent reviews and books on protecting groups, see:
- 9a K. Jarowicki, P. J. Kocienski, J. Chem. Soc. Perkin Trans. 1 2000, 2495–2527, and references therein;
- 9b J. R. Hanson, Protecting Groups in Organic Synthesis, Sheffield Academic Press, Sheffield, 1999;
- 9c
T. W. Greene, P. G. M. Wuts, Protective Groups in Organic Synthesis, 3rd ed., Wiley, New York, 1999;
10.1002/0471220574 Google Scholar
- 9d
M. Schelhaas, H. Waldmann, Angew. Chem. 1996, 108, 2192–2219;
10.1002/ange.19961081805 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2056–2083;
- 9e P. J. Kocienski, Protecting Groups, Georg Thieme, Stuttgart, 1994.
- 10
- 10a S.-C. Hung, S. R. Thopate, F.-C. Chi, S.-W. Chang, J.-C. Lee, C.-C. Wang, Y.-S. Wen, J. Am. Chem. Soc. 2001, 123, 3153–3154;
- 10b N. Komatsu, Tetrahedron Lett. 2001, 42, 1733–1736;
- 10c K. Fukase, Y. Fukase, M. Oikawa, W.-C. Liu, Y. Suda, S. Kusumoto, Tetrahedron 1998, 54, 4033–4050;
- 10d S. Hatakeyama, M. Yoshida, T. Esumi, Y. Iwabuchi, H. Irie, T. Kawamoto, H. Yamada, M. Nishizawa, Tetrahedron Lett. 1997, 38, 7887–7890;
- 10e N. Komatsu, J.-Y. Ishida, H. Suzuki, Tetrahedron Lett. 1997, 38, 7219–7222;
- 10f S. Hatakeyama, T. Ikeda, H. Irie, C. Izumi, H. Mori, K. Uenoyama, H. Yamada, M. Nishizawa, J. Chem. Soc. Chem. Commun. 1995, 1959–1960;
- 10g S. Hatekeyama, H. Mori, K. Kitano, H. Yamada, M. Nishizawa, Tetrahedron Lett. 1994, 35, 4367–4370;
- 10h T. Mukaiyama, K. Wariishi, M. Furuya, S. Kobayashi, Chem. Lett. 1989, 1277–1280;
- 10i M. B. Sassaman, K. D. Kotian, G. K. S. Prakash, G. A. Olah, J. Org. Chem. 1987, 52, 4314–4319;
- 10j S. Torii, S. Takagishi, T. Inokuchi, H. Okumoto, Bull. Chem. Soc. Jpn. 1987, 60, 775–776;
- 10k G. A. Olah, T. Yamato, P. S. Iyer, G. K. S. Prakash, J. Org. Chem. 1986, 51, 2826–2828;
- 10l J.-I. Kato, N. Iwasawa, T. Mukaiyama, Chem. Lett. 1985, 743–746;
- 10m T. Tsunoda, M. Suzuki, R. Noyori, Tetrahedron Lett. 1979, 4679–4680.
- 11
- 11a J. J. Gridley, A. J. Hacking, H. M. I. Osborn, D. G. Spackman, Tetrahedron 1998, 54, 14 925–14 946;
- 11b L. Jiang, T.-H. Chan, J. Org. Chem. 1998, 63, 6035–6038;
- 11c H. Qin, T. B. Grindley, J. Carbohydr. Chem. 1996, 15, 95–108;
- 11d D. J. Jenkins, B. V. L. Potter, Carbohydr. Res. 1994, 265, 145–149;
- 11e P. J. Garegg, I. Kvarnström, A. Niklasson, G. Niklasson, S. T. Svensson, J. Carbohydr. Chem. 1993, 12, 933–953;
- 11f L. Panza, S. Brasca, S. Riva, G. Russo, Tetrahedron: Asymmetry 1993, 4, 931–932;
- 11g B. Classon, P. J. Garegg, S. Oscarson, A.-K. Tidén, Carbohydr. Res. 1991, 216, 187–196;
- 11h S. Kim, H. Chang, W. J. Kim, J. Org. Chem. 1985, 50, 1751–1752;
- 11i R. Eby, K. T. Webster, C. Schuerch, Carbohydr. Res. 1984, 129, 111–120.
- 12 L. Jicsinszky, É. Fenyvesi, H. Hashimoto, A. Ueno in Comprehensive Supramolecular Chemistry, Vol. 3 ( ), Pergamon, Oxford, 1996, pp. 57–188.
- 13 M. Bukowska, M. Maciejewski, J. Prejzner, Carbohydr. Res. 1998, 308, 275–279.
- 14
- 14a R. Eby, C. Schuerch, Carbohydr. Res. 1982, 100, C41-C43;
- 14b H. M. Flowers, Carbohydr. Res. 1982, 100, 418–423.
- 15 R. R. Schmidt, W. Kinzy, Adv. Carbohydr. Chem. Biochem. 1994, 50, 21–121.
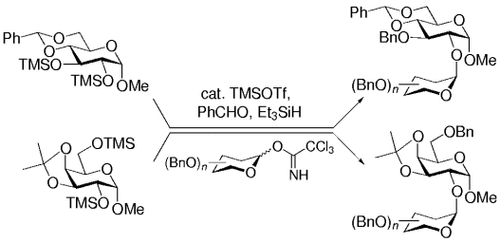
- 16 General Procedure: A mixture of the trimethylsilyl ether (1.0 equiv), freshly dried molecular sieves (3 Å, 1 mg per 1 mg trimethylsilyl ether), benzaldehyde (1.2 equiv), triethylsilane (1.2 equiv), and dichloromethane (8.5 mL per 1 mmol trimethylsilyl ether) was stirred at room temperature for 30 min under nitrogen. The mixture was cooled to −78 °C, trimethylsilyl trifluoromethanesulfonate (0.1 equiv) was slowly added, and the reaction was monitored by TLC. After the starting material was totally consumed, a solution of the glycosyl trichloroacetimidate[15] (1.2 equiv) in dichloromethane (5 mL per 1 mmol glycosyl donor) and trimethylsilyl trifluoromethanesulfonate (0.3 equiv) were added successively, the system was gradually warmed up to −40 °C, and the mixture was stirred at the same temperature overnight. The reaction was quenched with saturated aqueous sodium bicarbonate, and the aqueous phase was extracted with ethyl acetate (3×). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered, and evaporated in vacuo. The residue was purified by flash column chromatography to give the expected disaccharide. The yields are summarized in Schemes 1 and 2 .



41:13<>1.0.co;2-h.cover.gif)
