Characterization of a Δ8-Sphingolipid Desaturase from Higher Plants: A Stereochemical and Mechanistic Study on the Origin of E,Z Isomers†
Christoph Beckmann Dipl.-Chem.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorJanine Rattke
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorNeil J. Oldham Dr.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorPetra Sperling Dr.
Institut für Allgemeine Botanik, Universität Hamburg Ohnhorststr. 18, 22609 Hamburg (Germany)
Search for more papers by this authorErnst Heinz Prof. Dr.
Institut für Allgemeine Botanik, Universität Hamburg Ohnhorststr. 18, 22609 Hamburg (Germany)
Search for more papers by this authorWilhelm Boland Prof. Dr.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorChristoph Beckmann Dipl.-Chem.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorJanine Rattke
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorNeil J. Oldham Dr.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorPetra Sperling Dr.
Institut für Allgemeine Botanik, Universität Hamburg Ohnhorststr. 18, 22609 Hamburg (Germany)
Search for more papers by this authorErnst Heinz Prof. Dr.
Institut für Allgemeine Botanik, Universität Hamburg Ohnhorststr. 18, 22609 Hamburg (Germany)
Search for more papers by this authorWilhelm Boland Prof. Dr.
Max-Planck-Institut für Chemische Ökologie Winzerlaer Str. 10, 07745 Jena (Germany) Fax: (+49) 3641-571-202
Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft (SFB 436) and the Fonds der Chemischen Industrie for financial support.
Graphical Abstract
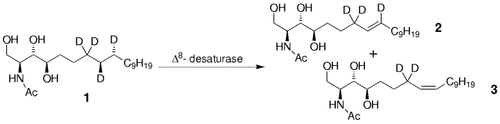
The simultaneous formation of E and Z double bonds results from the syn elimination of H and/or D atoms from different conformations of 4-hydroxysphinganine [D4]1. Δ8-Sphingolipid desaturase from Helianthus annuus is heterologously expressed in yeast and catalyzes the transformation to E olefin 2 (88%) and Z olefin 3 (12%).
References
- 1 A. H. Merill, J. Sweeley, C. C. Sweeley, in Biochemistry of lipids, lipoproteins and membranes ( ), Elsevier Science, Amsterdam, 1996.
- 2 C. K. Y. Ng, K. Carr, M. R. McAinsh, B. Powell, A. M. Hetherington, Nature 2001, 410, 596.
- 3 H. Imai, M. Ohnishi, K. Hotsubo, M. Kojima, S. Ito, Biosci. Biotechnol. Biochem. 1997, 61, 351.
- 4
J. A. Broadwater, J. A. Haas, B. G. Fox, Fett/Lipid 1998, 100, 103.
10.1002/(SICI)1521-4133(19985)100:4/5<103::AID-LIPI103>3.0.CO;2-4 CAS Web of Science® Google Scholar
- 5 P. Sperling, E. Heinz, Eur. J. Lipid Sci. Technol. 2001, 103, 158.
- 6 J. Shanklin, E. B. Cahoon, Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 611.
- 7 L. Fauconnot, P. H. Buist, J. Org. Chem. 2001, 66, 1210.
- 8 L. J. Morris, R. V. Harris, W. Kelly, A. T. James, Biochem. J. 1968, 109, 673.
- 9 Y. Lindqvist, W. J. Huang, G. Schneider, J. Shanklin, EMBO J. 1996, 15, 4081.
- 10 J. Du Bois, T. J. Mizoguchi, S. J. Lippard, Coord. Chem. Rev. 2000, 200, 443.
- 11 Y. S. Yang, J. Baldwin, B. A. Ley, J. M. Bollinger, E. I. Solomon, J. Am. Chem. Soc. 2000, 122, 8495.
- 12 D. Meesapyodsuk, D. W. Reed, C. K. Savile, P. H. Buist, S. J. Ambrose, P. S. Covello, Biochemistry 2000, 39, 11 948.
- 13 P. H. Buist, B. Behrouzian, J. Am. Chem. Soc. 1998, 120, 871.
- 14 P. H. Buist, B. Behrouzian, J. Am. Chem. Soc. 1996, 118, 6295.
- 15 A. Pinilla, F. Camps, G. Fabrias, Biochemistry 1999, 38, 15 272.
- 16
J. L. Abad, F. Camps, G. Fabrias, Angew. Chem. 2000, 112, 3417;
10.1002/1521-3757(20000915)112:18<3417::AID-ANGE3417>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3279.10.1002/1521-3773(20000915)39:18<3279::AID-ANIE3279>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 17 B. Behrouzian, P. H. Buist, J. Shanklin, Chem. Commun. 2001, 401.
- 18 A. Svatos, B. Kalinova, W. Boland, Insect Biochem. Mol. Biol. 1999, 29, 225.
- 19 I. Navarro, I. Font, G. Fabrias, F. Camps, J. Am. Chem. Soc. 1997, 119, 11 335.
- 20 W. Boland, C. Frössl, M. Schöttler, M. Toth, J. Chem. Soc. Chem. Commun. 1993, 1155.
- 21 J. L. Abad, F. Camps, G. Fabrias, Insect Biochem. Mol. Biol. 2001, 31, 799.
- 22 P. Sperling, A. Blume, U. Zähringer, E. Heinz, Biochem. Soc. Trans. 2000, 28, 638.
- 23 P. Sperling, U. Zähringer, E. Heinz, J. Biol. Chem. 1998, 273, 28 590.
- 24 F. Schneider, R. Lessire, J. J. Bessoule, H. Juguelin, C. Cassagne, Biochim. Biophys. Acta 1993, 1152, 243.
- 25 O. Thum, C. Hertweck, H. Simon, W. Boland, Synthesis 1999, 2145.
- 26 C. E. Tucker, P. Knochel, J. Org. Chem. 1993, 58, 4781.
- 27 H. C. Kolb, Van Nieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483.
- 28 T. Takata, R. Tajima, W. Ando, J. Org. Chem. 1983, 48, 4764.
- 29 Oxidative degradation of [7,7,9-D3]-(8E)-phytosphingenine yields a mixture of decanal and [1-D]decanal. The ratio of labeled and unlabeled decanal corresponds to the ratio of labeled and natural (E)-4-hydroxy-8-sphingenine prior to the cleavage.
- 30 C6-labeled palmitic acid yields the E/Z isomers of 4-hydroxy-8-sphingenine in a ratio of about 6:1, whereas the C7-labeled equivalent gives a lower ratio of the Z isomer (ca. 11:1) as a result of high KIE.
- 31 B. Behrouzian, L. Fauconnot, F. Daligault, C. Nugier-Chauvin, H. Patin, P. H. Buist, Eur. J. Biochem. 2001, 268, 3545.
- 32 C. K. Savile, G. Fabrias, P. H. Buist, J. Am. Chem. Soc. 2001, 123, 4382.



41:13<>1.0.co;2-h.cover.gif)
