B-Allyl-9-borabicyclo[3.3.1]nonane
Abstract
[53317-08-1] C11H19B (MW 162.07)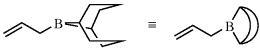
InChI = 1S/C11H19B/c1-2-9-12-10-5-3-6-11(12)8-4-7-10/h2,10-11H,1,3-9H2/t10-,11-
InChIKey = VWDZBSSQLJNTMJ-XYPYZODXSA-N
(allylating reagent for aldehydes, ketones, acid chlorides, amides, and acid anhydrides;2 reagent for diastereo- and enantioselective allylation of aldimines;6, 8 diastereoselective allylation of acetals9)
Alternate Name: allyl-9-BBN.
Physical Data: bp 41.5–42 °C/0.05 mmHg.
Solubility: sol most organic solvents.
Preparative Methods: prepared from Allyl Bromide and B-methoxy-9-BBN according to a literature procedure.1 Crotyl-9-BBN and 2-methallyl-9-BBN are similarly prepared from the corresponding allylic bromides.
Handling, Storage, and Precautions: the neat liquid is highly flammable and must be stored in the absence of air and moisture. Allyl-9-BBN may be kept as a CH2Cl2 or THF solution under N2 atmosphere.2 Use in a fume hood.



