The impact of the first, second and third waves of covid-19 on hepatitis B and C testing in Ontario, Canada
Abstract
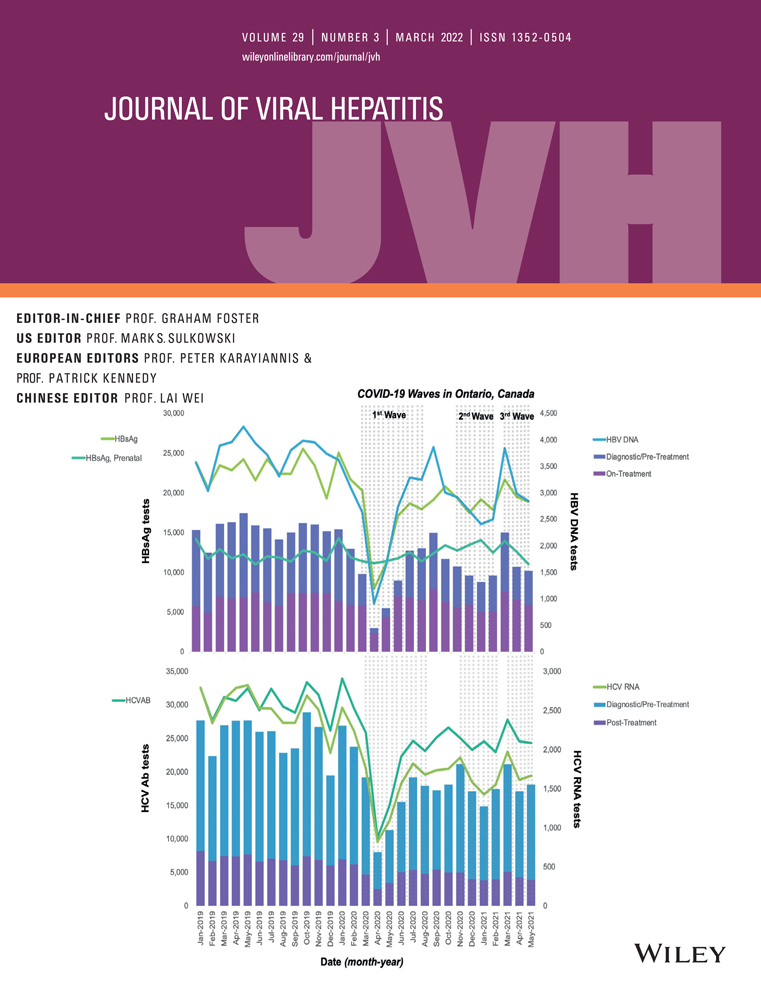
The COVID-19 pandemic interrupted routine healthcare services. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are often asymptomatic, and therefore, screening and on/post-treatment monitoring are required. Our aim was to determine the effect of the first, second and third waves of the pandemic on HBV and HCV testing in Ontario, Canada. We extracted data from Public Health Ontario for HBV and HCV specimens from 1 January 2019 to 31 May 2021. Testing volumes were evaluated and stratified by age, sex and region. Changes in testing volumes were analysed by per cent and absolute change. Testing volumes decreased in April 2020 with the first wave of the pandemic and recovered to 72%–75% of prepandemic volumes by the end of the first wave. HBsAg testing decreased by 33%, 18% and 15%, and HBV DNA testing decreased by 37%, 27% and 20%, in each consecutive wave. Anti-HCV testing decreased by 35%, 21% and 19%, and HCV RNA testing decreased by 44%, 30% and 36% in each consecutive wave. These trends were consistent by age, region and sex. Prenatal HBV testing volumes were stable. In conclusion, significant decreases in HBV and HCV testing occurred during the first three waves of the pandemic and have not recovered. In addition to direct consequences on viral hepatitis elimination efforts, these data provide insight into the impacts of the pandemic on chronic disease screening and management. Strategies to make up for missed testing will be critical to avoid additional consequences of COVID-19 long after the pandemic has resolved.
CONFLICT OF INTEREST
HS reports receiving research support from AbbVie, Intercept, Merck, Gilead Sciences and BMS. HLAJ reports receiving research support and/or consulting fees from Aligos, AbbVie, Arbutus, Arena, Bristol Myers Squibb, Eiger, Enyo, Gilead Sciences, Janssen, GlaxoSmithKline, Janssen, Merck, Regulus, Roche, VBI Vaccines, Vir. Biotechnology, Inc., and Viroclinics. MJB reports receiving research support and/or consulting fees from AbbVie, Gilead Sciences and Specialty Rx Solutions. JJF reports receiving research support and/or consulting fees from AbbVie, Antios, Enanta, Eiger, Gilead Sciences, Janssen, Roche, Arbutus, Vir and GlaxoSmithKline. No other competing interests were declared.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Public Health Ontario Laboratories. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the author(s) with the permission of Public Health Ontario Laboratories.