Update on B-cell maturation antigen-directed therapies in AL amyloidosis
Corresponding Author
Krzysztof Jamroziak
Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland
Correspondence
Krzysztof Jamroziak, Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Email: [email protected]
Search for more papers by this authorKlaudia Zielonka
Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorJahanzaib Khwaja
Department of Haematology, University College London Hospital, London, UK
Search for more papers by this authorAshutosh D. Wechalekar
Department of Haematology, University College London Hospital, London, UK
Search for more papers by this authorCorresponding Author
Krzysztof Jamroziak
Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland
Correspondence
Krzysztof Jamroziak, Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Email: [email protected]
Search for more papers by this authorKlaudia Zielonka
Department of Hematology, Transplantation and Internal Medicine, Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorJahanzaib Khwaja
Department of Haematology, University College London Hospital, London, UK
Search for more papers by this authorAshutosh D. Wechalekar
Department of Haematology, University College London Hospital, London, UK
Search for more papers by this authorKrzysztof Jamroziak and Klaudia Zielonka contributed equally to this work.
Summary
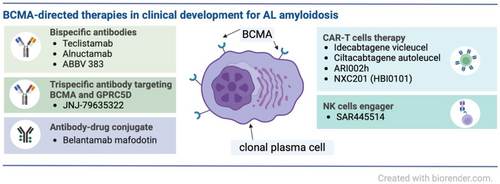
Systemic light chain (AL) amyloidosis is a rare clonal plasma cell disorder characterized by the production of amyloidogenic immunoglobulin light chains, which causes the formation and deposition of amyloid fibrils, leading to multi-organ dysfunction. Current treatment is directed at the underlying plasma cell clone to achieve a profound reduction in the monoclonal free light chain production. The standard-of-care first-line therapy is a combination of daratumumab, cyclophosphamide, bortezomib and dexamethasone (D-VCd regimen), resulting in high rates of haematological and organ responses. However, AL amyloidosis remains incurable, and all patients inevitably relapse. Hence, novel treatment options are needed for patients with an inadequate response or relapsed/refractory disease. B-cell maturation antigen (BCMA) is a tumour necrosis factor (TNF receptor superfamily receptor overexpressed on plasma cells in multiple myeloma (MM) and AL amyloidosis. Recently, several novel anti-BCMA immunotherapies have been approved for the treatment of relapsed/refractory MM, including antibody–drug conjugate belantamab mafodotin, bispecific antibodies teclistamab and elranatamab and chimeric antigen receptor T-cell therapies idecabtagene vicleucel and ciltacabtagene autoleucel. Despite lower expression than in MM, BCMA is also a promising target in AL amyloidosis. This review aims to provide up-to-date information on the efficacy and toxicity of anti-BCMA therapy in AL amyloidosis.
Graphical Abstract
Despite the significant progress in the treatment of systemic light chain (AL) amyloidosis with the introduction of the anti-CD38 monoclonal antibody daratumumab to the first-line therapy, relapsed or refractory disease remains an unmet medical need. B-cell maturation antigen (BCMA) is overexpressed on the surface of clonal plasma cells in AL amyloidosis and, therefore, represents a potential target for immunotherapy. Recent results from small clinical trials and retrospective studies suggest high efficacy of anti-BCMA strategies in relapsed refractory AL amyloidosis, including therapies already approved for multiple myeloma (antibody–drug conjugates belantamab mafodotin, bispecific T-cell redirecting antibodies teclistamab and elranatamab and chimeric T-cell antigen receptor therapies idecabtagene vicleucel and ciltacabtagene autoleucel) as well as novel immunotherapies in early clinical development.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016; 387(10038): 2641–2654.
- 2Staron A, Zheng L, Doros G, Connors LH, Mendelson LM, Joshi T, et al. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J. 2021; 11(8): 139.
- 3Ravichandran S, Lachmann HJ, Wechalekar AD. Epidemiologic and survival trends in amyloidosis, 1987–2019. N Engl J Med. 2020; 382(16): 1567–1568.
- 4Wechalekar AD, Cibeira MT, Gibbs SD, Jaccard A, Kumar S, Merlini G, et al. Guidelines for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA working group. Amyloid. 2023; 30(1): 3–17.
- 5Palladini G, Kastritis E, Maurer MS, Zonder J, Minnema MC, Wechalekar AD, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020; 136(1): 71–80.
- 6Sanchorawala V, Boccadoro M, Gertz M, Hegenbart U, Kastritis E, Landau H, et al. Guidelines for high dose chemotherapy and stem cell transplantation for systemic AL amyloidosis: EHA-ISA working group guidelines. Amyloid. 2022; 29(1): 1–7.
- 7Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013; 31(34): 4319–4324.
- 8Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020; 21(2): 207–221.
- 9Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022; 387(6): 495–505.
- 10Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023; 29(9): 2259–2267.
- 11Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004; 103(2): 689–694.
- 12Madry C, Laabi Y, Callebaut I, Roussel J, Hatzoglou A, Le Coniat M, et al. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int Immunol. 1998; 10(11): 1693–1702.
- 13 European Myeloma Network. A Phase 2 Study of Belantamab Mafodotin in Patients with Relapsed or Refractory AL Amyloidosis (EMN27), Identifier: NCT04617925 [Internet]. 2023 [cited 2023 Mar 14]. Available from: https://clinicaltrials.gov/study/NCT04617925
- 14Khwaja J, Bomsztyk J, Atta M, Bygrave C, Forbes A, Durairaj S, et al. Real-world efficacy of single-agent belantamab mafodotin in relapsed systemic AL amyloidosis. Br J Haematol. 2024; 204: 1811–1815.
- 15Kastritis E, Palladini G, Dimopoulos MA, Jaccard A, Merlini G, Theodorakakou F, et al. Efficacy and safety of belantamab mafodotin monotherapy in patients with relapsed or refractory light chain amyloidosis: a phase 2 study by the European Myeloma Network. Blood. 2023; 142(Suppl 1): 4779.
10.1182/blood-2023-185861 Google Scholar
- 16Lebel E, Vainstein V, Milani P, Palladini G, Shragai T, Lavi N, et al. Belantamab mafodotin in relapsed/refractory AL amyloidosis-real-world multi-center experience and review of the literature. Acta Haematol. 2024; 1-16: 1–8.
- 17Zhang Y, Godara A, Pan S, Toskic D, Mann H, Sborov D, et al. Belantamab mafodotin in patients with relapsed/refractory AL amyloidosis with myeloma. Ann Hematol. 2022; 101(9): 2119–2121.
- 18Chakraborty R, Bhutani D, Maurer MS, Mohan M, Lentzsch S, D'Souza A. Safety and efficacy of teclistamab in systemic immunoglobulin light chain amyloidosis. Blood Cancer J. 2023; 13(1): 172.
- 19Stalker M, Garfall AL, Cohen AD, Vogl DT, Susanibar-Adaniya S, Djulbegovic M, et al. Safety and efficacy of teclistamab in patients with relapsed or refractory AL amyloidosis: a retrospective case series. Blood. 2023; 142: 2035.
- 20Mann H, Toskic D, Pak K, Willard P, Fogaren T, Comenzo R. Teclistamab induces rapid responses in patients with relapsed/refractory AL amyloidosis. Blood. 2023; 142: 6766.
- 21Forgeard N, Elessa D, Carpinteiro A, Belhadj K, Minnema M, Roussel M, et al. Teclistamab in relapsed or refractory AL amyloidosis: a multinational retrospective case series. Blood. 2024; 143(8): 734–737.
- 22Lebel E, Kfir-Erenfeld S, Asherie N, Grisariu S, Avni B, Elias S, et al. Feasibility of a novel academic anti-BCMA chimeric antigen receptor T-cell (CART) (HBI0101) for the treatment of relapsed and refractory AL amyloidosis. Blood. 2023; 142(Suppl 1): 538.
10.1182/blood-2023-186450 Google Scholar
- 23Goel U, Dima D, Davis J, Ahmed N, Shaikh H, Lochner J, et al. Safety and efficacy of B cell maturation antigen-directed CAR T-cell therapy in patients with relapsed/refractory multiple myeloma and concurrent light chain amyloidosis. Eur J Haematol. 2024; 113: 817–823.
- 24Das S, Ailawadhi S, Sher T, Roy V, Fernandez A, Parrondo RD. Anti-B cell maturation antigen chimeric antigen receptor T cell therapy for the treatment of AL amyloidosis and concurrent relapsed/refractory multiple myeloma: preliminary efficacy and safety. Curr Oncol. 2023; 30(11): 9627–9633.
- 25Oliver-Caldes A, Jimenez R, Espanol-Rego M, Cibeira MT, Ortiz-Maldonado V, Quintana LF, et al. First report of CART treatment in AL amyloidosis and relapsed/refractory multiple myeloma. J Immunother Cancer. 2021; 9(12):e003783.
- 26Leung N, Chapman JA, Bhatia S. First report of teclistamab in a patient with relapsed AL amyloidosis and multiple myeloma. EJHaem. 2023; 4: 1157–1159.
- 27Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013; 19(8): 2048–2060.
- 28O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004; 199(1): 91–98.
- 29Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000; 192(1): 129–135.
- 30Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000; 10(13): 785–788.
- 31Hatzoglou A, Roussel J, Bourgeade MF, Rogier E, Madry C, Inoue J, et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000; 165(3): 1322–1330.
- 32Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020; 13(1): 125.
- 33Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013; 14(3): 290–297.
- 34Rosenzweig M, Urak R, Walter M, Lim L, Sanchez JF, Krishnan A, et al. Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy. 2017; 19(7): 861–866.
- 35Kfir-Erenfeld S, Asherie N, Grisariu S, Avni B, Zimran E, Assayag M, et al. Feasibility of a novel academic BCMA-CART (HBI0101) for the treatment of relapsed and refractory AL amyloidosis. Clin Cancer Res. 2022; 28(23): 5156–5166.
- 36Bal S, Sigler A, Chan A, Chung DJ, Dogan A, Giralt SA, et al. First description of B cell maturation antigen expression in light chain amyloidosis. Blood. 2019; 134(Suppl 1): 5452.
10.1182/blood-2019-127332 Google Scholar
- 37Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, et al. Gamma-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015; 6: 7333.
- 38Ghermezi M, Li M, Vardanyan S, Harutyunyan NM, Gottlieb J, Berenson A, et al. Serum B-cell maturation antigen: a novel biomarker to predict outcomes for multiple myeloma patients. Haematologica. 2017; 102(4): 785–795.
- 39Visram A, Soof C, Rajkumar SV, Kumar SK, Bujarski S, Spektor TM, et al. Serum BCMA levels predict outcomes in MGUS and smoldering myeloma patients. Blood Cancer J. 2021; 11(6): 120.
- 40Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012; 158(6): 727–738.
- 41Godara A, Zhou P, Rosenthal B, Kugelmass A, Toskic D, Fogaren T, et al. B-cell maturation antigen (BCMA) in systemic light-chain amyloidosis (AL): association with disease activity and its modulation with gamma-secretase inhibition. Blood. 2019; 134(Suppl 1): 4409.
- 42Godara A, Zhou P, Kugelmass A, Ma X, Rosenthal B, Toskic D, et al. Presence of soluble and cell-surface B-cell maturation antigen in systemic light-chain amyloidosis and its modulation by gamma-secretase inhibition. Am J Hematol. 2020; 95(5): E110–E113.
- 43Chen H, Yu T, Lin L, Xing L, Cho SF, Wen K, et al. Gamma-secretase inhibitors augment efficacy of BCMA-targeting bispecific antibodies against multiple myeloma cells without impairing T-cell activation and differentiation. Blood Cancer J. 2022; 12(8): 118.
- 44Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN 3rd, et al. Gamma-secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol. 2023; 24(7): 811–822.
- 45Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014; 123(20): 3128–3138.
- 46Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012; 30(7): 631–637.
- 47Nooka AK, Cohen AD, Lee HC, Badros A, Suvannasankha A, Callander N, et al. Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma: final analysis of the DREAMM-2 trial. Cancer. 2023; 129(23): 3746–3760.
- 48Khwaja J, Bomsztyk J, Mahmood S, Wisniowski B, Shah R, Tailor A, et al. High response rates with single-agent belantamab mafodotin in relapsed systemic AL amyloidosis. Blood Cancer J. 2022; 12(9): 128.
- 49Anderson L. A Dose-Finding and Proof-of-Concept Phase 1/2a Study of Belantamab Mafodotin in Relapsed or Refractory AL Amyloidosis, Identifier: NCT05145816 [Internet]. 2024 [cited 2024 Feb 29]. Available from: https://clinicaltrials.gov/study/NCT05145816
- 50Kaufman J, Raab M, Richard S, Grosicki S, Takács I, Strassz A, et al. Abstract CT067: the anti-BCMA antibody-drug conjugate HDP-101 with a novel amanitin payload shows promising initial first in human results in relapsed multiple myeloma. Cancer Res. 2024; 84(7_Suppl):CT067.
10.1158/1538-7445.AM2024-CT067 Google Scholar
- 51Hungria V, Robak P, Hus M, Zherebtsova V, Ward C, Ho PJ, et al. Belantamab mafodotin, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2024; 391(5): 393–407.
- 52Dimopoulos MA, Beksac M, Pour L, Delimpasi S, Vorobyev V, Quach H, et al. Belantamab mafodotin, pomalidomide, and dexamethasone in multiple myeloma. N Engl J Med. 2024; 391(5): 408–421.
- 53Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020; 126(14): 3192–3201.
- 54Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020; 4(18): 4538–4549.
- 55Foureau DM, Bhutani M, Robinson M, Guo F, Pham D, Buelow B, et al. Ex vivo efficacy of BCMA-bispecific antibody TNB-383B in relapsed/refractory multiple myeloma. EJHaem. 2020; 1(1): 113–121.
- 56Buelow B, Choudry P, Clarke S, Dang K, Davison L, Aldred SF, et al. Pre-clinical development of TNB-383B, a fully human T-cell engaging bispecific antibody targeting BCMA for the treatment of multiple myeloma. J Clin Oncol. 2018; 36(15_Suppl): 8034.
10.1200/JCO.2018.36.15_suppl.8034 Google Scholar
- 57Buelow B, Pham D, Choudhry P, Dang K, Pratap P, Clarke S, et al. T cell engagement without cytokine storm: a novel Bcma x CD3 antibody killing myeloma cells with minimal cytokine secretion. Blood. 2017; 130(Suppl 1): 501.
- 58Lesokhin AM, Raje N, Gasparetto CJ, Walker J, Krupka HI, Joh T, et al. Phase I, open-label study to evaluate the safety, pharmacokinetic, pharmacodynamic, and clinical activity of PF-06863135, a B-cell maturation antigen/CD3 bispecific antibody, in patients with relapsed/refractory advanced multiple myeloma. Blood. 2018; 132: 3229.
- 59Wong SW, Bar N, Paris L, Hofmeister CC, Hansson M, Santoro A, et al. Alnuctamab (ALNUC; BMS-986349; CC-93269), a B-cell maturation antigen (BCMA) x CD3 T-cell engager (TCE), in patients (pts) with relapsed/refractory multiple myeloma (RRMM): results from a phase 1 first-in-human clinical study. Blood. 2022; 140(Suppl 1): 400–402.
10.1182/blood-2022-159009 Google Scholar
- 60D'Souza A, Shah N, Rodriguez C, Voorhees PM, Weisel K, Bueno OF, et al. A phase I first-in-human study of ABBV-383, a B-cell maturation antigen x CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2022; 40(31): 3576–3586.
- 61 An Open-Label Phase 1b Study Evaluating the Safety and Efficacy of ABBV-383 in AL Amyloidosis, Identifier: NCT06158854 [Internet]. 2023 [cited 2023 Dec 6]. Available from: https://clinicaltrials.gov/study/NCT06158854
- 62Lee HC, Bumma N, Richter JR, Dhodapkar MV, Hoffman JE, Suvannasankha A, et al. LINKER-MM1 study: linvoseltamab (REGN5458) in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023; 41(16_Suppl): 8006.
10.1200/JCO.2023.41.16_suppl.8006 Google Scholar
- 63 A Phase 1/2 Study of Linvoseltamab in Patients With Relapsed or Refractory Systemic Light Chain Amyloidosis, Identifier: NCT06292780 [Internet]. 2024 [cited 2024 Mar 5]. Available from: https://clinicaltrials.gov/study/NCT06292780
- 64 Phase 1b Dose Expansion Study of NXC-201 for the Treatment of Patients with Relapsed or Refractory AL Amyloidosis, Identifier: NCT06097832 [Internet]. 2023 [cited 2023 Oct 24]. Available from: https://clinicaltrials.gov/study/NCT06097832
- 65 Phase 1, First-in-Human, Dose Escalation Study of JNJ-79635322, a Trispecific Antibody, in Participants with Relapsed or Refractory Multiple Myeloma or Previously Treated AL Amyloidosis, Identifier: NCT05652335 [Internet]. 2024 [cited 2024 Feb 28]. Available from: https://clinicaltrials.gov/study/NCT05652335
- 66 First-in-Human, Open-Label Phase 1/2 Study to Investigate Safety and Efficacy of SAR445514, an NKcell Engager (NKCE) Targeting B-cell Maturation Antigen (BCMA) in Monotherapy in Participants with Relapsed/Refractory Multiple Myeloma (RRMM) and in Relapsed/Refractory Light-chain Amyloidosis (RRLCA), Identifier: NCT05839626 [Internet]. 2023 [cited 2023 Nov 18]. Available from: https://clinicaltrials.gov/study/NCT05839626
- 67Pillarisetti R, Yang D, Yao J, Smith M, Luistro L, Vulfson P, et al. Characterization of JNJ-79635322, a novel BCMAxGPRC5DxCD3 T-cell redirecting trispecific antibody, for the treatment of multiple myeloma. Blood. 2023; 142(Suppl 1): 456.
10.1182/blood-2023-174941 Google Scholar
- 68Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol. 2018; 15(1): 47–62.
- 69Morris EC, Stauss HJ. Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood. 2016; 127(26): 3305–3311.
- 70Harush O, Asherie N, Kfir-Erenfeld S, Adler G, Barliya T, Assayag M, et al. Preclinical evaluation and structural optimization of anti-BCMA CAR to target multiple myeloma. Haematologica. 2022; 107(10): 2395–2407.
- 71Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021; 384(8): 705–716.
- 72Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021; 398(10297): 314–324.
- 73Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018; 6(1): 56.
- 74Ganatra S, Carver JR, Hayek SS, Ky B, Leja MJ, Lenihan DJ, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC council perspectives. J Am Coll Cardiol. 2019; 74(25): 3153–3163.
- 75Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019; 380(18): 1726–1737.
- 76Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023; 41(6): 1265–1274.
- 77Parrondo RD, Majeed U, Sher T. Antibody-based immunotherapy for treatment of immunoglobulin light-chain amyloidosis. Br J Haematol. 2020; 191(5): 673–681.
- 78Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. 2018; 29(5): 585–601.
- 79Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018; 11(1): 141.
- 80Asherie N, Kfir-Erenfeld S, Avni B, Assayag M, Dubnikov T, Zalcman N, et al. Development and manufacture of novel locally produced anti-BCMA CAR T cells for the treatment of relapsed/refractory multiple myeloma: results from a phase I clinical trial. Haematologica. 2023; 108(7): 1827–1839.
- 81Perez-Amill L, Sune G, Antonana-Vildosola A, Castella M, Najjar A, Bonet J, et al. Preclinical development of a humanized chimeric antigen receptor against B cell maturation antigen for multiple myeloma. Haematologica. 2021; 106(1): 173–184.
- 82de Larrea CFl. Clinical Trial Using Humanized CART Directed Against BCMA (ARI0002h) in Patients with Relapsed/Refractory Multiple Myeloma to Proteasome Inhibitors, Immunomodulators and Anti-CD38 Antibody. Identifier: NCT04309981 [Internet]. 2023 [cited 2023 Aug 23]. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04309981
- 83Demaria O, Gauthier L, Debroas G, Vivier E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021; 51(8): 1934–1942.