Epidemiological and clinical features, therapeutic strategies and outcomes in patients with hyperhaemolysis: A systematic review
Corresponding Author
Jeremy W. Jacobs
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Correspondence
Jeremy W. Jacobs, 55 Park St, New Haven, CT 06511, USA.
Email: [email protected]
Search for more papers by this authorLaura D. Stephens
Department of Pathology, University of California San Diego, La Jolla, California, USA
Search for more papers by this authorElizabeth S. Allen
Department of Pathology, University of California San Diego, La Jolla, California, USA
Search for more papers by this authorThomas C. Binns
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Search for more papers by this authorGarrett S. Booth
Department of Pathology, Microbiology & Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJeanne E. Hendrickson
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Search for more papers by this authorMatthew S. Karafin
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
Search for more papers by this authorChristopher A. Tormey
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Search for more papers by this authorJennifer S. Woo
Department of Pathology, City of Hope National Medical Center, Irvine, California, USA
Search for more papers by this authorBrian D. Adkins
Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Search for more papers by this authorCorresponding Author
Jeremy W. Jacobs
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Correspondence
Jeremy W. Jacobs, 55 Park St, New Haven, CT 06511, USA.
Email: [email protected]
Search for more papers by this authorLaura D. Stephens
Department of Pathology, University of California San Diego, La Jolla, California, USA
Search for more papers by this authorElizabeth S. Allen
Department of Pathology, University of California San Diego, La Jolla, California, USA
Search for more papers by this authorThomas C. Binns
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Search for more papers by this authorGarrett S. Booth
Department of Pathology, Microbiology & Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Search for more papers by this authorJeanne E. Hendrickson
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Search for more papers by this authorMatthew S. Karafin
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
Search for more papers by this authorChristopher A. Tormey
Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut, USA
Search for more papers by this authorJennifer S. Woo
Department of Pathology, City of Hope National Medical Center, Irvine, California, USA
Search for more papers by this authorBrian D. Adkins
Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Search for more papers by this authorJeremy W. Jacobs and Laura D. Stephens contributed equally to this study.
Summary
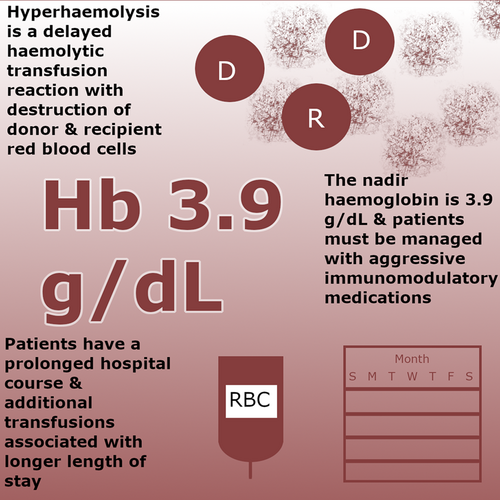
Hyperhaemolysis syndrome (HHS), a severe form of delayed haemolytic transfusion reaction most commonly described in patients with sickle cell disease (SCD), involves destruction of both donor and recipient red blood cells (RBCs). As the epidemiology and underlying pathophysiology have yet to be definitively elucidated, recognition can be challenging. We systematically reviewed PubMed and EMBASE to identify all cases of post-transfusion hyperhaemolysis and characterized the epidemiological, clinical and immunohaematological characteristics and treatments of HHS. We identified 51 patients (33 females and 18 males), including 31 patients with SCD (HbSS, HbSC and HbS/β-thalassaemia). The median haemoglobin nadir (3.9 g/dL) occurred a median of 10 days post-transfusion. 32.6% and 45.7% of patients had a negative indirect anti-globulin test and a negative direct anti-globulin test, respectively. The most common therapies included corticosteroids and intravenous immune globulin. 66.0% of patients received ≥1 supportive transfusion, which was associated with a longer median hospital stay/time to recovery (23 days vs. 15 days; p = 0.015) compared to no supportive transfusion. These findings illustrate that HHS that often results in marked anaemia 10 days post-transfusion is not restricted to patients with haemoglobinopathies, and additional transfused RBCs may be associated with a longer time-to-recovery.
Graphical Abstract
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest related to this research. All authors have no relevant disclosures.
Open Research
DATA AVAILABILITY STATEMENT
For original data, please contact [email protected].
Supporting Information
| Filename | Description |
|---|---|
| bjh18825-sup-0001-TableS1.docxWord 2007 document , 21 KB |
Table S1. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Ackfeld T, Schmutz T, Guechi Y, Le Terrier C. Blood transfusion reactions-a comprehensive review of the literature including a swiss perspective. J Clin Med. 2022; 11(10): 2859. Published 2022 May 19. https://doi.org/10.3390/jcm11102859
- 2Marik PE. Transfusion of blood and blood products. Evidence-Based Crit Care. 2014; 585–619. https://doi.org/10.1007/978-3-319-11020-2_38
10.1007/978?3?319?11020?2_38 Google Scholar
- 3 US Food & Drug Administration. Fatalities reported to FDA following blood collection and transfusion. https://www.fda.gov/media/160859/download#:~:text=During%20FY%202016%20there%20were,2019%2C%20and%2029%20in%202020 Accessed on February 7, 2023.
- 4Danaee A, Inusa B, Howard J, Robinson S. Hyperhemolysis in patients with hemoglobinopathies: a single-center experience and review of the literature. Transfus Med Rev. 2015; 29(4): 220–30. https://doi.org/10.1016/j.tmrv.2015.06.001
- 5Habibi A, Mekontso-Dessap A, Guillaud C, Michel M, Razazi K, Khellaf M, et al. Delayed hemolytic transfusion reaction in adult sickle-cell disease: presentations, outcomes, and treatments of 99 referral center episodes. Am J Hematol. 2016; 91(10): 989–94. https://doi.org/10.1002/ajh.24460
- 6Babb A, Diamantos N, Sekhar M. Hyperhaemolysis syndrome treated with corticosteroids and darbopoietin in a patient with mantle cell lymphoma. Transfus Med. 2012; 22(2): 142–4. https://doi.org/10.1111/j.1365-3148.2011.01095.x
- 7Cannas G, Dubreuil L, Fichez A, Gerfaud-Valentin M, Debard AL, Hot A. Delayed severe hemolytic transfusion reaction during pregnancy in a woman with β-thalassemia intermediate: successful outcome after eculizumab administration. Am J Case Rep. 2021; 22:e931107. https://doi.org/10.12659/AJCR.931107
- 8Gupta S, Fenves A, Nance ST, Sykes DB, Dzik WS. Hyperhemolysis syndrome in a patient without a hemoglobinopathy, unresponsive to treatment with eculizumab. Transfusion. 2015; 55(3): 623–8. https://doi.org/10.1111/trf.12876
- 9Win N. Hyperhemolysis syndrome in sickle cell disease. Expert Rev Hematol. 2009; 2(2): 111–5. https://doi.org/10.1586/ehm.09.2
- 10Epstein SS, Hadley TJ. Successful management of the potentially fatal hyperhaemolysis syndrome of sickle cell anaemia with a regimen including bortezomib and Hemopure. J Clin Pharm Ther. 2019; 44(5): 815–8. https://doi.org/10.1111/jcpt.12998
- 11Karafin MS, Singavi A, Johnson ST, Field JJ. A fatal case of immune hyperhemolysis with bone marrow necrosis in a patient with sickle cell disease. Hematol Rep. 2017; 9(1): 8–11. https://doi.org/10.4081/hr.2017.6934
10.4081/hr.2017.6934 Google Scholar
- 12Hendrickson JE, Fasano RM. Management of hemolytic transfusion reactions. Hematology Am Soc Hematol Educ Program. 2021; 2021(1): 704–9. https://doi.org/10.1182/hematology.2021000308
- 13Banks M, Shikle J. Hyperhemolysis syndrome in patients with sickle cell disease. Arch Pathol Lab Med. 2018; 142(11): 1425–7. https://doi.org/10.5858/arpa.2017-0251-RS
- 14Fuja C, Kothary V, Carll TC, Singh S, Mansfield P, Wool GD. Hyperhemolysis in a patient with sickle cell disease and recent SARS-CoV-2 infection, with complex auto- and alloantibody work-up, successfully treated with tocilizumab. Transfusion. 2022; 62(7): 1446–51. https://doi.org/10.1111/trf.16932
- 15Conrado MCAV, Fonseca GSVC, Dezan MR, Mendes FR, Hamasaki DT, Chinoca KZ, et al. Accelerated erythrocyte destruction mimicking post-transfusion hyperhaemolysis in the course of uncomplicated vaso-occlusive crisis associated with sickle cell disease. Transfus Med. 2020; 30(6): 522–4. https://doi.org/10.1111/tme.12717
- 16Chou ST, Alsawas M, Fasano RM, Field JJ, Hendrickson JE, Howard J, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020; 4(2): 327–55. https://doi.org/10.1182/bloodadvances.2019001143
- 17Petz LD, Garratty G. Immune hemolytic anemias. Philadelphia: Churchill Livingstone; 2004.
- 18Marouf R. Blood transfusion in sickle cell disease. Hemoglobin. 2011; 35(5–6): 495–502. https://doi.org/10.3109/03630269.2011.596984
- 19Adkins BD, Sharma D, Eichbaum Q. Can we better predict delayed hemolytic transfusion reactions and hyperhemolysis in sickle cell disease? Transfus Apher Sci. 2020; 59(2):102681. https://doi.org/10.1016/j.transci.2019.102681
- 20Test ST, Woolworth VS. Defective regulation of complement by the sickle erythrocyte: evidence for a defect in control of membrane attack complex formation. Blood. 1994; 83(3): 842–52.
- 21Götze O, Müller-Eberhard HJ. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970; 132(5): 898–915. https://doi.org/10.1084/jem.132.5.898
- 22Lachmann PJ, Thompson RA. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970; 131(4): 643–57. https://doi.org/10.1084/jem.131.4.643
- 23Tormey CA, Hendrickson JE. Transfusion-related red blood cell alloantibodies: induction and consequences. Blood. 2019; 133(17): 1821–30. https://doi.org/10.1182/blood-2018-08-833962
- 24Mwesigwa S, Moulds JM, Chen A, Flanagan J, Sheehan VA, George A, et al. Whole-exome sequencing of sickle cell disease patients with hyperhemolysis syndrome suggests a role for rare variation in disease predisposition. Transfusion. 2018; 58(3): 726–35. https://doi.org/10.1111/trf.14431
- 25Roumenina LT, Bartolucci P, Pirenne F. The role of complement in post-transfusion hemolysis and hyperhemolysis reaction. Transfus Med Rev. 2019; 33(4): 225–30. https://doi.org/10.1016/j.tmrv.2019.09.007
- 26Chonat S, Mener A, Verkerke H, Stowell SR. Role of complement in alloimmunization and hyperhemolysis. Curr Opin Hematol. 2020; 27(6): 406–14. https://doi.org/10.1097/MOH.0000000000000610
- 27 Soliris. https://alexion.com/Documents/Soliris_USPI.pdf Accessed on February 4, 2023.
- 28Win N, Lucas S, Hebballi S, McKernan A, Hamilton R, Robinson I, et al. Histopathological evidence for macrophage activation driving post-transfusion hyperhaemolysis syndrome. Br J Haematol. 2019; 186(3): 499–502. https://doi.org/10.1111/bjh.15925
- 29Lee LE, Beeler BW, Graham BC, Cap AP, Win N, Chen F. Posttransfusion hyperhemolysis is arrested by targeting macrophage activation with novel use of tocilizumab. Transfusion. 2020; 60(1): 30–5. https://doi.org/10.1111/trf.15562
- 30Chadebech P, Habibi A, Nzouakou R, Bachir D, Meunier-Costes N, Bonin P, et al. Delayed hemolytic transfusion reaction in sickle cell disease patients: evidence of an emerging syndrome with suicidal red blood cell death. Transfusion. 2009; 49(9): 1785–92. https://doi.org/10.1111/j.1537-2995.2009.02199.x
- 31Win N, Yeghen T, Needs M, Chen FE, Okpala I. Use of intravenous immunoglobulin and intravenous methylprednisolone in hyperhaemolysis syndrome in sickle cell disease. Hematology. 2004; 9(5–6): 433–6. https://doi.org/10.1080/10245330400001926
- 32 Actemra. https://www.gene.com/download/pdf/actemra_prescribing.pdf Accessed on February 6, 2023.
- 33Sivapalaratnam S, Linpower L, Sirigireddy B, Agapidou A, Jain S, Win N, et al. Treatment of post-transfusion hyperhaemolysis syndrome in sickle cell disease with the anti-IL6R humanised monoclonal antibody tocilizumab. Br J Haematol. 2019; 186(6): e212–4. https://doi.org/10.1111/bjh.16103
- 34Menakuru SR, Priscu A, Dhillon V, Salih A. Acute hyperhemolysis syndrome in a patient with known sickle cell anemia refractory to steroids and IVIG treated with tocilizumab and erythropoietin: a case report and review of literature. Hematol Rep. 2022; 14(3): 235–9. https://doi.org/10.3390/hematolrep14030032
- 35Wu Y, Ji Y, Dai B, Guo F, Wu Y, He Z, et al. A case of hyperhaemolysis syndrome in a pregnant Chinese woman with β-thalassemia during perinatal transfusion. Transfus Med. 2021; 31(1): 24–9. https://doi.org/10.1111/tme.12748
- 36Nickel RS, Hendrickson JE, Fasano RM, Meyer EK, Winkler AM, Yee MM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016; 56(1): 107–14. https://doi.org/10.1111/trf.13379