The impact of prenatal alcohol and/or tobacco exposure on brain structure in a large sample of children from a South African birth cohort
Andrew T. Marshall
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorStefanie C. Bodison
Department of Occupational Therapy, College of Public Health and Health Professions, University of Florida, Gainesville, Florida, USA
Search for more papers by this authorKristina A. Uban
Department of Public Health, University of California, Irvine, California, USA
Search for more papers by this authorShana Adise
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorDeborah Jonker
Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorWeslin Charles
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorKirsten A. Donald
Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorEric Kan
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorJonathan C. Ipser
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorLetitia Butler-Kruger
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorBabette Steigelmann
Maastricht University, Maastricht, The Netherlands
Search for more papers by this authorKatherine L. Narr
Department of Neurology, UCLA Brain Mapping Center, Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA
Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorShantanu H. Joshi
Department of Neurology, UCLA Brain Mapping Center, Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA
Department of Bioengineering, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorLucy T. Brink
Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorHein J. Odendaal
Department of Obstetrics and Gynaecology, Stellenbosch University, Cape Town, South Africa
Search for more papers by this authorFreda Scheffler
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorDan J. Stein
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Unit on Risk and Resilience in Mental Disorders, South African Medical Research Council (SAMRC), Cape Town, South Africa
Search for more papers by this authorCorresponding Author
Elizabeth R. Sowell
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Correspondence
Elizabeth R. Sowell, Department of Pediatrics, Children's Hospital Los Angeles, 4650 Sunset Blvd., Mailstop #130, Los Angeles, CA 90027, USA.
Email: [email protected]
Search for more papers by this authorAndrew T. Marshall
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorStefanie C. Bodison
Department of Occupational Therapy, College of Public Health and Health Professions, University of Florida, Gainesville, Florida, USA
Search for more papers by this authorKristina A. Uban
Department of Public Health, University of California, Irvine, California, USA
Search for more papers by this authorShana Adise
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorDeborah Jonker
Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorWeslin Charles
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorKirsten A. Donald
Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorEric Kan
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Search for more papers by this authorJonathan C. Ipser
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorLetitia Butler-Kruger
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorBabette Steigelmann
Maastricht University, Maastricht, The Netherlands
Search for more papers by this authorKatherine L. Narr
Department of Neurology, UCLA Brain Mapping Center, Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA
Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorShantanu H. Joshi
Department of Neurology, UCLA Brain Mapping Center, Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA
Department of Bioengineering, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorLucy T. Brink
Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, California, USA
Search for more papers by this authorHein J. Odendaal
Department of Obstetrics and Gynaecology, Stellenbosch University, Cape Town, South Africa
Search for more papers by this authorFreda Scheffler
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Search for more papers by this authorDan J. Stein
Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Neuroscience Institute, University of Cape Town, Cape Town, South Africa
Unit on Risk and Resilience in Mental Disorders, South African Medical Research Council (SAMRC), Cape Town, South Africa
Search for more papers by this authorCorresponding Author
Elizabeth R. Sowell
Department of Pediatrics, Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, California, USA
Correspondence
Elizabeth R. Sowell, Department of Pediatrics, Children's Hospital Los Angeles, 4650 Sunset Blvd., Mailstop #130, Los Angeles, CA 90027, USA.
Email: [email protected]
Search for more papers by this authorDan J. Stein and Elizabeth R. Sowell co-senior authors.
Abstract
Background
Neuroimaging studies have emphasized the impact of prenatal alcohol exposure (PAE) on brain development, traditionally in heavily exposed participants. However, less is known about how naturally occurring community patterns of PAE (including light to moderate exposure) affect brain development, particularly in consideration of commonly occurring concurrent impacts of prenatal tobacco exposure (PTE).
Methods
Three hundred thirty-two children (ages 8 to 12) living in South Africa's Cape Flats townships underwent structural magnetic resonance imaging. During pregnancy, their mothers reported alcohol and tobacco use, which was used to evaluate PAE and PTE effects on their children's brain structure. Analyses involved the main effects of PAE and PTE (and their interaction) and the effects of PAE and PTE quantity on cortical thickness, surface area, and volume.
Results
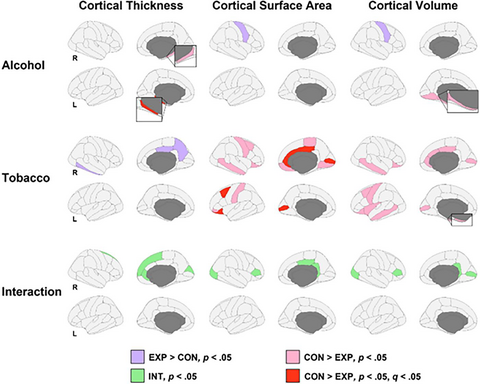
After false-discovery rate (FDR) correction, PAE was associated with thinner left parahippocampal cortices, while PTE was associated with smaller cortical surface area in the bilateral pericalcarine, left lateral orbitofrontal, right posterior cingulate, right rostral anterior cingulate, left caudal middle frontal, and right caudal anterior cingulate gyri. There were no PAE × PTE interactions nor any associations of PAE and PTE exposure on volumetrics that survived FDR correction.
Conclusion
PAE was associated with reduction in the structure of the medial temporal lobe, a brain region critical for learning and memory. PTE had stronger and broader associations, including with regions associated with executive function, reward processing, and emotional regulation, potentially reflecting continued postnatal exposure to tobacco (i.e., second-hand smoke exposure). These differential effects are discussed with respect to reduced PAE quantity in our exposed group versus prior studies within this geographical location, the deep poverty in which participants live, and the consequences of apartheid and racially and economically driven payment practices that contributed to heavy drinking in the region. Longer-term follow-up is needed to determine potential environmental and other moderators of the brain findings here and assess the extent to which they endure over time.
Graphical Abstract
We analyzed brain structure of 332 8- to 12-year-old children from the Prenatal Alcohol, SIDS and Stillbirth (PASS) Network who had varying levels of prenatal alcohol (PAE) and/or tobacco exposure (PTE). PAE was associated with thinner parahippocampal cortices; PTE’s impact was more regionally expansive. Such PAE and PTE effects reflect highly complex downstream outcomes, which require consideration in terms of the amount of exposure, the deep poverty in which participants live, and the legacy of the dop system.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting Information
| Filename | Description |
|---|---|
| acer14945-sup-0001-TableS1-S8.docxWord 2007 document , 161.6 KB |
Tables S1-S8 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- Adebiyi, B.O., Mukumbang, F.C. & Beytell, A.-M. (2021) Policy requirements for the prevention and management of fetal alcohol spectrum disorder in South Africa: a policy brief. Frontiers in Public Health, 9, 592726.
- Astley, S.J. & Clarren, S.K. (2001) Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol and Alcoholism, 36, 147–159.
- Baer, J.S., Sampson, P.D., Barr, H.M., Connor, P.D. & Streissguth, A.P. (2003) A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. JAMA Psychiatry, 60, 377–385.
- Benjamini, Y. & Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300.
- Bhattacharya, D., Fujihashi, A., Majrashi, M., Bloemer, J., Bhattacharya, S., Buabeid, M. et al. (2020) Concurrent nicotine exposure to prenatal alcohol consumption alters the hippocampal and cortical neurotoxicity. Heliyon, 6, e03045.
- Brink, L.T., Nel, D.G., Hall, D.R. & Odendaal, H.J. (2020) Association of socioeconomic status and clinical and demographic conditions with the prevalence of preterm birth. International Journal of Gynecology & Obstetrics, 149, 359–369.
- Brooks-Gunn, J. & Duncan, G.J. (1997) The effects of poverty on children. The Future of Children, 7, 55–71.
- Chastain, L.G. & Sarkar, D.K. (2017) Alcohol effects on the epigenome in the germline: role in the inheritance of alcohol-related pathology. Alcohol, 60, 53–66.
- Cornelius, M.D. & Day, N.L. (2009) Developmental consequences of prenatal tobacco exposure. Current Opinion in Neurology, 22, 121–125.
- Cornelius, M.D., Ryan, C.M., Day, N.L., Goldschmidt, L. & Willford, J.A. (2001) Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental and Behavioral Pediatrics, 22, 217–225.
- De Guio, F., Mangin, J.-F., Rivière, D., Perrot, M., Molteno, C.D., Jacobson, S.W. et al. (2014) A study of cortical morphology in children with fetal alcohol spectrum disorders. Human Brain Mapping, 35, 2285–2296.
- Dhupelia-Mesthrie, U. (2014) Speaking about building Rylands (1960s to 1980s): a cape flats history. Social Dynamics, 40, 353–370.
- Donald, K.A., Eastman, E., Howells, F.M., Adnams, C., Riley, E.P., Woods, R.P. et al. (2015) Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatrica, 27, 251–269.
- Donald, K.A., Roos, A., Fouche, J.-P., Koen, N., Howells, F.M., Woods, R.P. et al. (2015) A study of the effects of prenatal alcohol exposure on white matter microstructural integrity at birth. Acta Neuropsychiatrica, 27, 197–205.
- Dukes, K.A., Burd, L., Elliott, A.J., Fifer, W.P., Folkerth, R.D., Hankins, G.D.V. et al. (2014) The safe passage study: design, methods, recruitment, and follow-up approach. Paediatric and Perinatal Epidemiology, 28, 455–465.
- Dukes, K., Tripp, T., Petersen, J., Robinson, F., Odendaal, H., Elliot, A. et al. (2017) A modified timeline Followback assessment to capture alcohol exposure in pregnant women: application in the safe passage study. Alcohol, 62, 17–27.
- Dukes, K., Tripp, T., Willinger, M., Odendaal, H., Elliott, A.J., Kinney, H.C. et al. (2017) Drinking and smoking patterns during pregnancy: development of group-based trajectories in the safe passage study. Alcohol, 62, 49–60.
- El Marroun, H., Schmidt, M.N., Franken, I.H.A., Jaddoe, V.W.V., Hofman, A., Van Der Lugt, A. et al. (2014) Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology, 39, 792–800.
- El Marroun, H., Tiemeier, H., Franken, I.H.A., Jaddoe, V.W.V., Van Der Lugt, A., Verhulst, F.C. et al. (2016) Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biological Psychiatry, 79, 971–979.
- Elliott, A.J., Kinney, H.C., Haynes, R.L., Dempers, J.D., Wright, C., Fifer, W.P. et al. (2020) Concurrent prenatal drinking and smoking increases risk for Sids: safe passage study report. EclinicalMedicine, 19, 100247.
- Ernst, M., Moolchan, E.T. & Robinson, M.L. (2001) Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry, 40, 630–641.
- Fan, J., Jacobson, S.W., Taylor, P.A., Molteno, C.D., Dodge, N.C., Stanton, M.E. et al. (2016) White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Human Brain Mapping, 37, 2943–2958.
- Fischl, B., Salat, D.H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C. et al. (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355.
- Fischl, B., Salat, D.H., Van Der Kouwe, A.J.W., Makris, N., Ségonne, F., Quinn, B.T. et al. (2004) Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23, S69–S84.
- Gautam, P., Lebel, C., Narr, K.L., Mattson, S.N., May, P.A., Adnams, C.M. et al. (2015) Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Human Brain Mapping, 36, 2318–2329.
- Gonzalez, M.R., Palmer, C.E., Uban, K.A., Jernigan, T.L., Thompson, W.K. & Sowell, E.R. (2020) Positive economic, psychosocial, and physiological ecologies predict brain structure and cognitive performance in 9-10-year-old children. Frontiers in Human Neuroscience, 14, 578822.
- Hellemans, K.G.C., Sliwowska, J.H., Verma, P. & Weinberg, J. (2010) Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience & Biobehavioral Reviews, 34, 791–807.
- Huizink, A.C. & Mulder, E.J.H. (2006) Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Reviews, 30, 24–41.
- Jacobs, L. & Jacobs, J. (2013) Narratives on alcohol dependence in the family in post-apartheid South Africa. Journal of Addiction Research & Therapy, 4, 1000152.
10.4172/2155-6105.1000152 Google Scholar
- Jacobson, S.W., Jacobson, J.L., Molteno, C.D., Warton, C.M.R., Wintermark, P., Hoyme, H.E. et al. (2017) Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn Mri scans. Alcoholism: Clinical and Experimental Research, 41, 965–975.
- Joseph, J., Warton, C., Jacobson, S.W., Jacobson, J.L., Molteno, C.D., Eicher, A. et al. (2014) Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders. Human Brain Mapping, 35, 659–672.
- Kraev, T.A., Adamkiewicz, G., Hammond, S.K. & Spengler, J.D. (2009) Indoor concentrations of nicotine in low-income multi-unit housing: associations with smoking behaviours and housing characteristics. Tobacco Control, 18, 438–444.
- Lange, S., Probst, C., Quere, M., Rehm, J. & Popova, S. (2015) Alcohol use, smoking and their co-occurrence during pregnancy among Canadian women, 2003 to 2011/12. Addictive Behaviors, 50, 102–109.
- Lange, S., Probst, C., Rehm, J. & Popova, S. (2018) National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. The Lancet Global Health, 6, E769–E776.
- Laube, C., Van Den Bos, W. & Fandakova, Y. (2020) The relationship between pubertal hormones and brain plasticity: implications for cognitive training in adolescence. Developmental Cognitive Neuroscience, 42, 100753.
- Lebel, C., Mattson, S.N., Riley, E.P., Jones, K.L., Adnams, C.M., May, P.A. et al. (2012) A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. The Journal of Neuroscience, 34, 15243–15251.
- Lees, B., Mewton, L., Jacobus, J., Valadez, E.A., Stapinski, L.A., Teesson, M. et al. (2020) Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the adolescent brain cognitive development study. The American Journal of Psychiatry, 177, 1060–1072.
- Lees, B., Mewton, L., Stapinski, L.A., Teesson, M. & Squeglia, L.M. (2020) Association of prenatal alcohol exposure with preadolescent alcohol sipping in the Abcd study®. Drug and Alcohol Dependence, 214, 108187.
- Li, Y. & Wang, H. (2004) In utero exposure to tobacco and alcohol modifies neurobehavioral development in mice offspring: consideration a role of oxidative stress. Pharmacological Research, 49, 467–473.
- Lindinger, N.M., Jacobson, J.L., Warton, C.M.R., Malcolm-Smith, S., Molteno, C.D., Dodge, N.C. et al. (2021) Fetal alcohol exposure alters bold activation patterns in brain regions mediating the interpretation of facial affect. Alcoholism: Clinical and Experimental Research, 45, 140–152.
- London, L. (2000) Alcohol consumption amongst south African farm workers: a challenge for post-apartheid health sector transformation. Drug and Alcohol Dependence, 59, 199–206.
- Louw, J.G., Van Heerden, A., Olivier, L., Lambrechts, T., Broodryk, M., Bunge, L. et al. (2021) Executive function after prenatal alcohol exposure in children in a south African population: cross-sectional study. JMIR Formative Research, 5, e20658.
- Mattson, S.N., Bernes, G.A. & Doyle, L.R. (2019) Fetal alcohol spectrum disorders: a review of neurobehavioral deficits associated with prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 43, 1046–1062.
- May, P.A., Baete, A., Russo, J., Elliott, A.J., Blankenship, J., Kalberg, W.O. et al. (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics, 134, 855–866.
- May, P.A., Blankenship, J., Marais, A.-S., Gossage, J.P., Kalberg, W.O., Barnard, R. et al. (2013) Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a south African population-based study. Alcoholism: Clinical and Experimental Research, 37, 818–830.
- May, P.A., Marais, A.-S., De Vries, M., Hasken, J.M., Stegall, J.M., Hedrick, D.M. et al. (2019) The Dop system of alcohol distribution is dead, but it's legacy lives on…. International Journal of Environmental Research and Public Health, 16, 3701.
- Miles, M., Warton, F.L., Meintjes, E.M., Molteno, C.D., Jacobson, J.L., Jacobson, S.W. et al. (2021) Effects of prenatal alcohol exposure on the volumes of the lateral and medial walls of the intraparietal sulcus. Frontiers in Neuroanatomy, 15, 639800.
- Moore, E.M., Migliorini, R., Infante, M.A. & Riley, E.P. (2014) Fetal alcohol spectrum disorders: recent neuroimaging findings. Current Developmental Disorders Reports, 1, 161–172.
- Moore, E.M. & Xia, Y. (2021) Neurodevelopmental trajectories following prenatal alcohol exposure. Frontiers in Human Neuroscience, 15, 695855.
- Mowinckel, A. M. & Vidal-Piñeiro, D. (2019). Visualisation of brain statistics with R-packages ggseg and ggseg3d. arXiv preprint arXiv:1912.08200.
- Nadhiroh, S.R., Djokosujono, K. & Utari, D.M. (2020) The association between secondhand smoke exposure and growth outcomes of children: a systematic literature review. Tobacco Induced Diseases, 18, 12.
- Noble, K.G., Houston, S.M., Brito, N.H., Bartsch, H., Kan, E., Kuperman, J.M. et al. (2015) Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778.
- Nuñez, S.C., Roussotte, F. & Sowell, E.R. (2011) Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Research & Health, 34, 121–132.
- Odendaal, H.J., Kruger, M. & Botha, M.H. (2020) Dangers of smoking cigarettes and drinking alcohol during pregnancy. South African Medical Journal, 110, 1066–1067.
- Oh, S., Gonzalez, J.M.R., Salas-Wright, C.P., Vaughn, M.G. & Dinitto, D.M. (2017) Prevalence and correlates of alcohol and tobacco use among pregnant women in the United States: evidence from the Nsduh 2005–2014. Preventative Medicine, 97, 93–99.
- Polli, F.S. & Kohlmeier, K.A. (2020) Prenatal nicotine exposure in rodents: why are there so many variations in behavioral outcomes? Nicotine & Tobacco Research, 22, 1694–1710.
- Reuter, M., Rosas, H.D. & Fischl, B. (2010) Highly accurate inverse consistent registration: A robust approach. NeuroImage, 53, 1181–1196.
- Riley, E.P., Infante, M.A. & Warren, K.R. (2011) Fetal alcohol spectrum disorders: an overview. Neuropsychology Review, 21, 73–80.
- Roffman, J.L., Sipahi, E.D., Dowling, K.F., Hughes, D.E., Hopkinson, C.E., Lee, H. et al. (2021) Association of adverse prenatal exposure burden with child psychopathology in the adolescent brain cognitive development (Abcd) study. PLoS One, 16, e0250235.
- Sabde, Y. & Zodpey, S. (2011) Secondhand tobacco smoke exposure in low income group women of Nagpur, India. Asian Journal of Experimental Sciences, 25, 81–85.
- Ségonne, F., Dale, A.M., Busa, E., Glessner, M., Salat, D., Hahn, H.K. et al. (2004) A hybrid approach to the skull stripping problem in Mri. NeuroImage, 22, 1060–1075.
- Skagerström, J., Alehagen, S., Häggström-Nordin, E., Årestedt, K. & Nilsen, P. (2013) Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: a cross sectional study in Sweden. BMC Public Health, 13, 780.
- Sled, J.G., Zijdenbos, A.P. & Evans, A.C. (1998) A nonparametric method for automatic correction of intensity nonuniformity in Mri data. IEEE Transactions on Medical Imaging, 17, 87–97.
- Sowell, E.R., Mattson, S.N., Thompson, P.M., Jernigan, T.L., Riley, E.P. & Toga, A.W. (2001) Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology, 57, 235–244.
- Sulik, K.K. (1984) Critical periods for alcohol teratogenesis in mice, with special reference to the gastrulation stage of embryogenesis. Ciba Foundation Symposium, 105, 124–141.
- Sulik, K.K. (2005) Genesis of alcohol-induced craniofacial dysmorphism. Experimental Biology and Medicine, 230, 366–375.
- Sulik, K.K., Johnston, M.C., Daft, P.A., Russell, W.E., Dehart, D.B., Opitz, J.M. et al. (1986) Fetal alcohol syndrome and DiGeorge anomaly: critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. American Journal of Medical Genetics, 25, 97–112.
10.1002/ajmg.1320250614 Google Scholar
- Turok, I., Visagie, J. & Scheba, A. (2021) Social inequality and spatial segregation in Cape Town. In: M. Ham, T. Tammaru, R. Ubarevičienė & H. Janssen (Eds.) Urban socio-economic segregation and income inequality. Switzerland: Springer.
10.1007/978-3-030-64569-4_4 Google Scholar
- Uban, K.A., Jonker, D., Donald, K.A., Brooks, S.J., Bodison, S.C., Kan, E. et al. (2022) Associations between prenatal alcohol and tobacco exposure and cortical and subcortical brain measures in south African children: a pilot study. medRxiv. Available from: https://doi.org/10.1101/2022.06.07.22276078
10.1101/2022.06.07.22276078 Google Scholar
- Wade, N.E., Palmer, C.E., Gonzalez, M.R., Wallace, A.L., Infante, M.A., Tapert, S.F. et al. (2021) Risk factors associated with curiosity about alcohol use in the Abcd cohort. Alcohol, 92, 11–19.
- Wickham, H., Chang, W., Henry, L., Pedersen, T.L., Takahashi, K., Wilke, C. et al. (2016) ggplot2: elegant graphics for data analysis. New York: Springer-Verlag.
10.1007/978-3-319-24277-4 Google Scholar
- Wilkinson, P. (2000) City profile: Cape Town. Cities, 17, 195–205.