Can the Enhanced Liver Fibrosis Score Be Used to Diagnose Children With Liver Fibrosis?
Sources of Funding: The assays used for measuring the components of the Enhanced Liver Fibrosis test were provided without restrictions by Siemens Healthcare A/S (Ballerup, Denmark). The study was funded by the Odense University Hospital Free Research Fund, the Augustinus Foundation and the Danish Kidney association Research Foundation.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
An infographic is available for this article at: http://links.lww.com/MPG/C535.
ABSTRACT
Objectives:
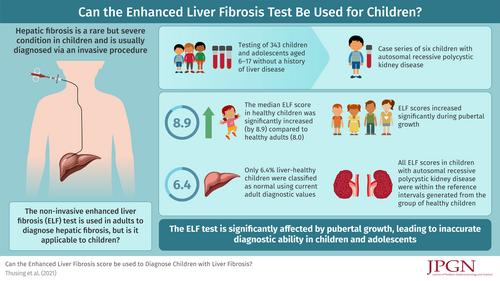
The noninvasive Enhanced Liver Fibrosis (ELF) score is used in adults with liver fibrosis as a diagnostic aid. The ELF score combines 3 serum markers of extracellular matrix remodeling and fibrogenesis: hyaluronic acid (HA), the N-terminal pro-peptide of collagen type III (PIIINP), and tissue inhibitor of metalloproteinase-1 (TIMP-1). We aimed to evaluate the clinical use of the ELF score in children.
Methods and Results:
A reference interval for the ELF score was established using 343 liver-healthy children ages 6 to 17 years. The median ELF score of 8.9 in healthy children was significantly increased compared with healthy adults. ELF scores increased significantly in both female and male healthy controls with peak levels at puberty, driven by elevated levels of HA and PIIINP likely explained by increased growth. If adult normal values were applied to the group of liver-healthy children, only 6.4% were in the normal range. Prospectively, we analysed ELF scores in patients with possible or confirmed liver fibrosis because of autosomal recessive polycystic kidney disease (ARPKD). All ELF scores in children with ARPKD were within the reference intervals generated from the group of healthy children.
Conclusions:
The usual diagnostic cut-off ranges for the ELF score in adults are not applicable; instead age and gender-appropriate cut-off values should be used in children. The clinical value of ELF scores in children is questionable as children during pubertal growth showed elevated ELF scores and patients with ARPKD and liver fibrosis showed normal levels.