Synthesen, Kristallstrukturen und Drillingsbildung der Cluster-Trimere Bi2[PtBi6Br12]3 und Bi2[PtBi6I12]3†
Professor Hubert Langbein zum 65. Geburtstag gewidmet
Abstract
Syntheses, Crystal Structures, and Triple Twinning of the Cluster Trimers Bi2[PtBi6Br12]3 and Bi2[PtBi6I12]3
Melting reactions of Bi with Pt and BiX3 (X = Br, I) yield shiny black, air insensitive crystals of the subhalides Bi2[PtBi6X12]. Bi2[PtBi6Br12]3 crystallizes in the monoclinic space group C2/m with lattice parameters a = 1617.6(2) pm, b = 1488.5(1) pm, c = 1752.4(2) pm, and β = 110.85(4)°. Bi2[PtBi6I12]3 adopts the triclinic space group 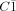 with pseudo-monoclinic lattice parameters a = 1711.2(2) pm, b = 1585.1(1) pm, c = 1865.7(2) pm, and α = 90°, β = 111.15(4)°, γ = 90°. The two homoeotypic compounds consist of cuboctahedral [Pt−IIBiII6X−I12]2− clusters that are concatenated into linear trimers by BiIII atoms. The ordered distribution of BiIII atoms destroys the inherent threefold rotation axes in the packing of cluster anions. As a consequence of the pseudosymmetry the crystals are triple twinned along [201]. Due to different orientations of the cluster trimers there are two BiII···X inter-cluster bridges per BiII atom in Bi2[PtBi6Br12]3 but only one bridge in Bi2[PtBi6I12]3. The structure of the iodine compound can be deduced from the NaCl structure type, leaving 37 of 96 atomic positions unoccupied. The arrangement of the cuboctahedral clusters follows the motif of a body-centered cubic packing.
with pseudo-monoclinic lattice parameters a = 1711.2(2) pm, b = 1585.1(1) pm, c = 1865.7(2) pm, and α = 90°, β = 111.15(4)°, γ = 90°. The two homoeotypic compounds consist of cuboctahedral [Pt−IIBiII6X−I12]2− clusters that are concatenated into linear trimers by BiIII atoms. The ordered distribution of BiIII atoms destroys the inherent threefold rotation axes in the packing of cluster anions. As a consequence of the pseudosymmetry the crystals are triple twinned along [201]. Due to different orientations of the cluster trimers there are two BiII···X inter-cluster bridges per BiII atom in Bi2[PtBi6Br12]3 but only one bridge in Bi2[PtBi6I12]3. The structure of the iodine compound can be deduced from the NaCl structure type, leaving 37 of 96 atomic positions unoccupied. The arrangement of the cuboctahedral clusters follows the motif of a body-centered cubic packing.




