Facile Synthesis of Poly(3,4-ethylenedioxythiophene) Nanofibers from an Aqueous Surfactant Solution
Moon Gyu Han Dr.
Center for Optical Materials Science and Engineering Technology, School of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA, Fax: (+1) 864-656-1049
Search for more papers by this authorStephen H. Foulger Prof.
Center for Optical Materials Science and Engineering Technology, School of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA, Fax: (+1) 864-656-1049
Search for more papers by this authorMoon Gyu Han Dr.
Center for Optical Materials Science and Engineering Technology, School of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA, Fax: (+1) 864-656-1049
Search for more papers by this authorStephen H. Foulger Prof.
Center for Optical Materials Science and Engineering Technology, School of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA, Fax: (+1) 864-656-1049
Search for more papers by this authorGraphical Abstract
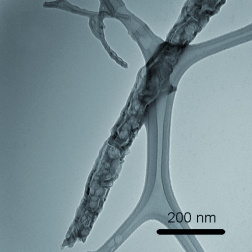
Easy does it: Poly(3,4-ethylenedioxythiophene) (PEDOT) fibers can be synthesized from aqueous solutions of anionic surfactant by a self-assembly method. This approach readily generates nanofibers with diameters down to about 10 nm and lengths of over 5 μm (see TEM image). The fibers can be produced with high yields and exhibit electrical conductivities of approximately 46 S cm−1.
References
- 1Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, H. Yan, Adv. Mater. 2003, 15, 353–389.
- 2C. R. Martin, Acc. Chem. Res. 1995, 28, 61–68.
- 3M. Law, J. Goldberger, P. Yang, Annu. Rev. Mater. Res. 2004, 34, 83–122.
- 4M. S. Dresselhaus, G. Dresselhaus, P. C. Eklund, Science of Fullerenes and Carbon Nanotubes, Academic Press, New York, 1996.
- 5A. G. MacDiarmid, Angew. Chem. 2001, 113, 2649–2659;
10.1002/1521-3757(20010716)113:14<2649::AID-ANGE2649>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2581–2590.10.1002/1521-3773(20010716)40:14<2581::AID-ANIE2581>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 6J. Wang, S. Chan, R. R. Carlson, Y. Luo, G. Ge, R. S. Ries, J. R. Heath, H. R. Tseng, Nano Lett. 2004, 4, 1693–1697.
- 7M. Granström, M. Berggren, O. Inganäs, Science 1995, 267, 1479–1481.
- 8X. Yang, J. Loos, S. C. Veenstar, W. J. H. Verhees, M. M. Wienk, J. M. Kroon, M. A. J. Michels, R. A. J. Janssen, Nano Lett. 2005, 5, 579–583.
- 9S. I. Cho, W. J. Kwon, S. J. Choi, P. Kim, S. A. Park, J. Kim, S. J. Son, R. Xiao, S. H. Kim, S. B. Lee, Adv. Mater. 2005, 17, 171–175.
- 10C. G. Wu, T. Bein, Science 1994, 264, 1757–1759.
- 11C. R. Martin, Science 1994, 266, 1961–1966.
- 12J. Huang, S. Virji, B. H. Weiller, R. B. Kaner, J. Am. Chem. Soc. 2003, 125, 314–315.
- 13X. Zhang, S. K. Manohar, J. Am. Chem. Soc. 2004, 126, 12714–12715.
- 14N.-R. Chiou, A. J. Epstein, Adv. Mater. 2005, 17, 1679–1683.
- 15Z. Wei, M. Wan, Adv. Mater. 2002, 14, 1314–1317.
- 16H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu, E. P. Woo, Science 2000, 290, 2123–2126.
- 17B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik, J. R. Reynolds, Adv. Mater. 2000, 12, 481–494.
- 18G. Heywang, F. Jonas, Adv. Mater. 1992, 4, 116–118.
- 19M. G. Han, S. H. Foulger, Adv. Mater. 2004, 16, 231–234.
- 20B. H. Kim, M. S. Kim, K. T. Park, J. K. Lee, D. H. Park, J. Joo, S. G. Yu, S. H. Lee, Appl. Phys. Lett. 2003, 83, 539–541.
- 21M. G. Han, S. P. Armes, Langmuir, 2003, 19, 4523–4526.
- 22M. G. Han, S. H. Foulger, Chem. Commun. 2004, 2154–2155.
- 23X. Zhang, A. G. MacDiarmid, S. K. Manohar, Chem. Commun. 2005, 5328–5330.
- 24J. Jang, M. Chang, H. Yoon, Adv. Mater. 2005, 17, 1620–1625.
- 25X. Zhang, J.-S. Lee, G. S. Lee, D.-K. Cha, M. J. Kim, D. J. Yang, S. K. Manohar, Macromolecules 2006, 39, 470–472.
- 26M. G. Han, S. H. Foulger, Chem. Commun. 2005, 3092–3094.
- 27M. J. Rosen, Surfactants and Interfacial Phenomena, 2nd. ed., Wiley, New York, 1988.
- 28J. Zhao, B. M. Fung, Langmuir 1993, 9, 1228–1231.
- 29S. Hayashi, S. Ikeda, J. Phys. Chem. 1980, 84, 744–751.
- 30J. Jang, J. Bae, Angew. Chem. 2004, 116, 3891–3894; Angew. Chem. Int. Ed. 2004, 43, 3803–3806.
- 31N. A. Mazer, G. B. Benedek, M. C. Carey, J. Phys. Chem. 1976, 80, 1075–1085.
- 32R. Corradi, S. P. Armes, Synth. Met. 1997, 84, 453–454.
- 33J. W. Choi, M. G. Han, S. Y. Kim, S. G. Oh, S. S. Im, Synth. Met. 2004, 141, 293–299.
- 34P. J. Missel, N. A. Mazer, M. C. Carey, G. B. Benedek, J. Phys. Chem. 1989, 93, 8354–8366.
- 35B. H. Kim, D. H. Park, J. Joo, S. G. Yu, S. H. Lee, Synth. Met. 2005, 150, 279–284.
- 36B. J. Kim, S. G. Oh, M. G. Han, S. S. Im, Langmuir 2000, 16, 5841–5845.
- 37H. J. Ahonen, J. Lukkari, J. Kankare, Macromolecules 2000, 33, 6787–6793.
- 38D. Hohnholz, A. G. MacDiarmid, D. M. Sarno, W. E. Jones, Jr., Chem. Commun. 2001, 2444–2445.
- 39T. Johansson, L. A. A. Pettersson, O. Inganäs, Synth. Met. 2002, 129, 269–274.
- 40A. N. Aleshin, Adv. Mater. 2006, 18, 17–28.
- 41I. Winter, C. Reese, J. Hormes, G. Heywang, F. Jonas, Chem. Phys. 1995, 194, 207–213.
- 42N. Sakmeche, S. Aeiyach, J.-J. Aaron, M. Jouini, J. C. Lacroix, P.-C. Lacaze, Langmuir 1999, 15, 2566–2574.
- 43G. Greczynski, T. H. Kugler, M. Keil, W. Osikowicz, M. Fahlman, W. R. Salaneck, J. Electron Spectrosc. Relat. Phenom. 2000, 121, 1–17.
- 44G. Zotti, S. Zecchin, G. Schiavon, F. Louwet, L. Groenendaal, X. Crispin, W. Osikowicz, W. Salaneck, M. Fahlman, Macromolecules 2003, 36, 3337–3344.
- 45K. E. Aasmundtveit, E. J. Samuelson, L. A. A. Petterson, O. Inganäs, T. Johansson, R. Feidenhans’l, Synth. Met. 1999, 101, 561–564.
- 46T. Kawai, M. Nakazono, K. Yoshino, J. Mater. Chem. 1992, 2, 903–906.