Prostate DCE-MRI with  correction using an approximated analytical approach
correction using an approximated analytical approach
Corresponding Author
Xinran Zhong
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Correspondence Xinran Zhong, Department of Radiological Sciences, David Geffen School of Medicine, 300 UCLA Medical Plaza, Suite B114 Los Angeles, CA 90095. Email: [email protected]Search for more papers by this authorThomas Martin
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorHolden H. Wu
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorKrishna S. Nayak
Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, California
Search for more papers by this authorKyunghyun Sung
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorCorresponding Author
Xinran Zhong
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Correspondence Xinran Zhong, Department of Radiological Sciences, David Geffen School of Medicine, 300 UCLA Medical Plaza, Suite B114 Los Angeles, CA 90095. Email: [email protected]Search for more papers by this authorThomas Martin
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorHolden H. Wu
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorKrishna S. Nayak
Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, California
Search for more papers by this authorKyunghyun Sung
Department of Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, California
Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, California
Search for more papers by this authorAbstract
Purpose
To develop and evaluate a practical
 correction method for prostate dynamic contrast-enhanced (DCE) MRI analysis.
correction method for prostate dynamic contrast-enhanced (DCE) MRI analysis.
Theory
We proposed a simple analytical
 correction method using a Taylor series approximation to the steady-state spoiled gradient echo signal equation. This approach only requires
correction method using a Taylor series approximation to the steady-state spoiled gradient echo signal equation. This approach only requires
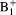 maps and uncorrected pharmacokinetic (PK) parameters as input to estimate the corrected PK parameters.
maps and uncorrected pharmacokinetic (PK) parameters as input to estimate the corrected PK parameters.
Methods
The proposed method was evaluated using a prostate digital reference object (DRO), and 82 in vivo prostate DCE-MRI cases. The approximated analytical correction was compared with the ground truth PK parameters in simulation, and compared with the reference numerical correction in in vivo experiments, using percentage error as the metric.
Results
The prostate DRO results showed that our approximated analytical approach provided residual error less than 0.4% for both Ktrans and ve, compared to the ground truth. This noise-free residual error was smaller than the noise-induced error using the reference numerical correction, which had a minimum error of 2.1+4.3% with baseline signal-to-noise ratio of 234.5. For the 82 in vivo cases, Ktrans and ve percentage error compared to the reference numerical correction method had a mean of 0.1% (95% central range of [0.0%, 0.2%]) across the prostate volume.
Conclusion
The approximated analytical
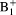 correction method provides comparable results with less than 0.2% error within 95% central range, compared to reference numerical
correction method provides comparable results with less than 0.2% error within 95% central range, compared to reference numerical
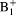 correction. The proposed method is a practical solution for
correction. The proposed method is a practical solution for
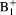 correction in prostate DCE-MRI because of its simple implementation.
correction in prostate DCE-MRI because of its simple implementation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
| Filename | Description |
|---|---|
| mrm27232-sup-0001-suppinfo01.docxWord document, 6.4 MB |
FIGURE S1. EA, DRO maps using 3 population-averaged AIFs for Ktrans estimation (a–c) and for ve estimation (d–f). The maximum residual error for Ktrans is 0.2% and for ve is 0.4%. FIGURE S2. Extended Tofts model was simulated in the DRO with 3 vp values, 0.001, 0.005, and 0.01. The results were evaluated using EA,DRO for Ktrans (a–c) and ve estimation (d–f). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30.
- 2Litjens G, Debats O, Barentsz J, Karssemeijer N, Huisman H. Computer-aided detection of prostate cancer in MRI. IEEE transactions on medical imaging. 2014; 33: 1083–1092.
- 3Isebaert S, De Keyzer F, Haustermans K, et al. Evaluation of semi-quantitative dynamic contrast-enhanced MRI parameters for prostate cancer in correlation to whole-mount histopathology. Eur J Radiol 2012; 81: e217–e222.
- 4Alonzi R, Padhani AR, Allen C. Dynamic contrast enhanced MRI in prostate cancer. Eur J Radiol 2007; 63: 335–350.
- 5Kamrava M, Kishan AU, Margolis DJ, et al. Multiparametric magnetic resonance imaging for prostate cancer improves Gleason score assessment in favorable risk prostate cancer. Pract Radiat Oncol 2015; 5: 411–416.
- 6Khalifa F, Soliman A, El-Baz A, et al. Models and methods for analyzing DCE-MRI: a review. Med Phys 2014; 41: 124301.
- 7Franiel T, Hamm B, Hricak H. Dynamic contrast-enhanced magnetic resonance imaging and pharmacokinetic models in prostate cancer. Eur Radiol 2011; 21: 616–626.
- 8Vos EK, Litjens GJ, Kobus T, et al. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur Urol 2013; 64: 448–455.
- 9Fennessy FM, McKay RR, Beard CJ, Taplin ME, Tempany CM. Dynamic contrast-enhanced magnetic resonance imaging in prostate cancer clinical trials: potential roles and possible pitfalls. Transl Oncol 2014; 7: 120–129.
- 10Tofts P. T1-weighted DCE imaging concepts: modelling, acquisition and analysis. Signal 2010; 500: 400.
- 11Gupta RK. A new look at the method of variable nutation angle for the measurement of spin-lattice relaxation times using fourier transform NMR. J Magn Reson 1977; 25: 231–235.
- 12Homer J, Beevers MS. Driven-equilibrium single-pulse observation of T1 relaxation. A reevaluation of a rapid “new” method for determining NMR spin-lattice relaxation times. J Magn Reson 1985; 63: 287–297.
- 13Treier R, Steingoetter A, Fried M, Schwizer W, Boesiger P. Optimized and combined T1 and B1 mapping technique for fast and accurateT1 quantification in contrast-enhanced abdominal MRI. Magn Reson Med 2007; 57: 568–576.
- 14Tsai WC, Kao KJ, Chang KM, et al. B1 field correction of T1 estimation should be considered for breast dynamic contrast-enhanced MR imaging even at 1.5 T. Radiology 2017; 282: 55–62.
- 15Subashi E, Choudhury KR, Johnson GA. An analysis of the uncertainty and bias in DCE-MRI measurements using the spoiled gradient-recalled echo pulse sequence. Med Phys 2014; 41: 032301.
- 16Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. Am J Roentgenol 2007; 189: W192–W201.
- 17Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am 2007; 15: 277–290.
- 18Rangwala NA, Dregely I, Wu HH, Sung K. Optimization and evaluation of reference region variable flip angle (RR-VFA) B1 + and T 1 mapping in the prostate at 3T. J Magn Reson Imaging 2017; 45: 751–760.
- 19Di Giovanni P, Azlan CA, Ahearn TS, Semple SI, Gilbert FJ, Redpath TW. The accuracy of pharmacokinetic parameter measurement in DCE-MRI of the breast at 3 T. Phys Med Biol 2010; 55: 121–132.
- 20Cunningham CH, Pauly JM, Nayak KS. Saturated double-angle method for rapidB1 + mapping. Magn Reson Med 2006; 55: 1326–1333.
- 21Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B 1 mapping by Bloch-Siegert shift. Magn Reson Med 2010; 63: 1315–1322.
- 22Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 2007; 57: 192–200.
- 23Sung K, Saranathan M, Daniel BL, Hargreaves BA. Simultaneous T 1 and B 1 + mapping using reference region variable flip angle imaging. Magn Reson Med 2013; 70: 954–961.
- 24Bedair R, Graves MJ, Patterson AJ, et al. Effect of radiofrequency transmit field correction on quantitative dynamic contrast-enhanced MR imaging of the breast at 3.0 T. Radiology 2016; 279: 368–377.
- 25Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997; 7: 91–101.
- 26Zhong X, Rangwala N, Raman S, Margolis D, Wu H, Sung K. B1 + inhomogeneity correction for estimation of pharmacokinetic parameters through an approximation approach. In Proceedings of the 24th Annual Meeting of ISMRM, 2016, Singapore. p. 2491.
- 27Bosca RJ, Jackson EF. Creating an anthropomorphic digital MR phantom—an extensible tool for comparing and evaluating quantitative imaging algorithms. Phys Med Biol 2016; 61: 974–982.
- 28Mehrtash A, Gupta SN, Shanbhag D, et al. Bolus arrival time and its effect on tissue characterization with dynamic contrast-enhanced magnetic resonance imaging. J Med Imaging 2016; 3: 14503.
- 29Zhong X, Wu H, Nayak K, Sung K. Evaluation of approximation method for B1 + correction using digital reference object in prostate DCE-MRI. In Proceedings of the 25th Annual Meeting of ISMRM, 2017, Honolulu, HI. p. 622.
- 30Coleman TF, Li Y. An interior trust region approach for nonlinear minimization subject to bounds. SIAM J Optim 1996; 6: 418–445.
- 31 QIBA Content. Daniel P. Barboriak Lab. https://sites.duke.edu/dblab/qibacontent/. 2012. Accessed June 8, 2016.
- 32Azahaf M, Haberley M, Betrouni N, et al. Impact of arterial input function selection on the accuracy of dynamic contrast-enhanced MRI quantitative analysis for the diagnosis of clinically significant prostate cancer. J Magn Reson Imaging 2016; 43: 737–749.
- 33Parker GJ, Roberts C, Macdonald A, et al. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med 2006; 56: 993–1000.
- 34Weinmann HJ, Laniado M, Mützel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Medical NMR 1984; 16: 167–172.
- 35Fritz-Hansen T, Rostrup E, Larsson HB, et al. Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med 1996; 36: 225–231.
- 36Peng Y, Jiang Y, Yang C, et al. Quantitative analysis of multiparametric prostate MR images: differentiation between prostate cancer and normal tissue and correlation with gleason score—a computer-aided diagnosis development study. Radiology 2013; 267: 787–796.
- 37Ma J. Breath-hold water and fat imaging using a dual-echo two-point dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med 2004; 52: 415–419.
- 38Orton MR, D'Arcy JA, Walker-Samuel S, et al. Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 2008; 53: 1225–1239.
- 39Portalez D, Mozer P, Cornud F, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol 2012; 62: 986–996.
- 40de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 2004; 230: 652–659.
- 41Chao SL, Metens T, Lemort M. TumourMetrics: a comprehensive clinical solution for the standardization of DCE-MRI analysis in research and routine use. Quant Imaging Med Surg 2017; 7: 1–15.
- 42Port RE, Knopp MV, Brix G. Dynamic contrast-enhanced MRI using Gd-DTPA: interindividual variability of the arterial input function and consequences for the assessment of kinetics in tumors. Magn Reson Med 2001; 45: 1030–1038.
- 43Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 1991; 17: 357–367.