Cerebral oxygen extraction fraction and cerebral venous blood volume measurements using MRI: Effects of magnetic field variation
Hongyu An
Department of Biomedical Engineering, Washington University, St. Louis, Missouri
Search for more papers by this authorCorresponding Author
Weili Lin
Department of Biomedical Engineering, Washington University, St. Louis, Missouri
Department of Radiology and Biomedical Engineering, University of North Carolina at Chapel Hill, North Carolina
The University of North Carolina at Chapel Hill, Department of Radiology, CB#7515, Chapel Hill, NC 27599===Search for more papers by this authorHongyu An
Department of Biomedical Engineering, Washington University, St. Louis, Missouri
Search for more papers by this authorCorresponding Author
Weili Lin
Department of Biomedical Engineering, Washington University, St. Louis, Missouri
Department of Radiology and Biomedical Engineering, University of North Carolina at Chapel Hill, North Carolina
The University of North Carolina at Chapel Hill, Department of Radiology, CB#7515, Chapel Hill, NC 27599===Search for more papers by this authorAbstract
The presence of magnetic background field inhomogeneity (ΔB) may confound quantitative measures of cerebral venous blood volume (vCBV) and cerebral oxygen extraction fraction (MR_OEF) with T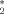 -based methods. The goal of this study was to correct its effect and obtain more accurate estimates of vCBV and MR_OEF. A 3D high-resolution gradient echo sequence was employed to obtain ΔB maps by two algorithms. The ΔB maps were then used to recover the signal loss in images acquired by a 2D multiecho gradient echo / spin echo sequence. Finally, both quantitative estimates of MR_OEF and vCBV were obtained from the ΔB- corrected 2D multiecho gradient echo / spin echo images. A total of 12 normal subjects were studied. An overestimated vCBV was observed in the brain (4.29 ± 0.78%) prior to ΔB correction, while the measured vCBV was substantially reduced after ΔB correction. Whole brain vCBV of 2.97 ± 0.44% and 2.68 ± 0.47% were obtained by the two different ΔB correction methods, in excellent agreement with the reported results in the literature. Furthermore, when MR_OEF was compared with and without ΔB correction, no significant differences (P = 0.467) were observed. The ability to simultaneously obtain vCBV and MR_OEF noninvasively may have profound clinical implications for the studies of cerebrovascular disease. Magn Reson Med 47:958–966, 2002. © 2002 Wiley-Liss, Inc.
-based methods. The goal of this study was to correct its effect and obtain more accurate estimates of vCBV and MR_OEF. A 3D high-resolution gradient echo sequence was employed to obtain ΔB maps by two algorithms. The ΔB maps were then used to recover the signal loss in images acquired by a 2D multiecho gradient echo / spin echo sequence. Finally, both quantitative estimates of MR_OEF and vCBV were obtained from the ΔB- corrected 2D multiecho gradient echo / spin echo images. A total of 12 normal subjects were studied. An overestimated vCBV was observed in the brain (4.29 ± 0.78%) prior to ΔB correction, while the measured vCBV was substantially reduced after ΔB correction. Whole brain vCBV of 2.97 ± 0.44% and 2.68 ± 0.47% were obtained by the two different ΔB correction methods, in excellent agreement with the reported results in the literature. Furthermore, when MR_OEF was compared with and without ΔB correction, no significant differences (P = 0.467) were observed. The ability to simultaneously obtain vCBV and MR_OEF noninvasively may have profound clinical implications for the studies of cerebrovascular disease. Magn Reson Med 47:958–966, 2002. © 2002 Wiley-Liss, Inc.
REFERENCES
- 1 Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 1982; 714: 265–270.
- 2 Ogawa S, Menon RS, Tank DW, Kim SG, and Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 1993; 64: 803–812.
- 3 Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imag 1991; 1: 275–283.
- 4 van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging [see Comments]. Nat Med 1998; 4: 159–167.
- 5 An H, Lin W. Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cerebr Blood Flow Metab 2000; 20: 1225–1236.
- 6 Lammertsma AA, Wise RJ, Heather JD, Gibbs JM, Leenders KL, Frackowiak RS, Rhodes CG, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain. 2. Results in normal subjects and brain tumour and stroke patients. J Cerebr Blood Flow Metab 1983; 3: 425–431.
- 7 Grubb RL, Phelps ME, Ter-Pogossian MM. Regional cerebral blood volume in humans. X-ray fluorescence studies. Arch Neurol 1973; 28: 38–44.
- 8 Sakai F, Nakazawa K, Tazaki Y, Ishii K, Hino H, Igarashi H, Kanda T. Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cerebr Blood Flow Metab 1985; 5: 207–213.
- 9 Perlmutter JS, Powers WJ, Herscovitch P, Fox PT, Raichle ME. Regional asymmetries of cerebral blood flow, blood volume, and oxygen utilization and extraction in normal subjects. J Cerebr Blood Flow Metab 1987; 7: 64–67.
- 10 Brooks DJ, Beaney RP, Leenders KL, Marshall J, Thomas DJ, Jones T. Regional cerebral oxygen utilization, blood flow, and blood volume in benign intracranial hypertension studied by positron emission tomography. Neurology 1985; 35: 1030–1034.
- 11 Leggett RW, Williams LR. Suggested reference values for regional blood volumes in humans. Health Phys 1991; 60: 139–154.
- 12 Kuppusamy K, Lin W, Cizek GR, Haacke EM. In vivo regional cerebral blood volume: quantitative assessment with 3D T1-weighted pre- and postcontrast MR imaging. Radiology 1996; 201: 106–112.
- 13 Lin W, Paczynski RP, Kuppusamy K, Hsu CY, Haacke EM. Quantitative measurements of regional cerebral blood volume using MRI in rats: effects of arterial carbon dioxide tension and mannitol. Magn Reson Med 1997; 38: 420–428.
- 14
Yang QX,
Williams GD,
Demeure RJ,
Mosher TJ,
Smith MB.
Removal of local field gradient artifacts in T
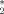 -weighted images at high fields by gradient-echo slice excitation profile imaging.
Magn Reson Med
1998;
39:
402–409.
-weighted images at high fields by gradient-echo slice excitation profile imaging.
Magn Reson Med
1998;
39:
402–409.
- 15 Reichenbach JR, Venkatesan R, Yablonskiy DA, Thompson MR, Lai S, Haacke EM. Theory and application of static field inhomogeneity effects in gradient-echo imaging. J Magn Reson Imag 1997; 7: 266–279.
- 16 Yablonskiy DA. Quantitation of intrinsic magnetic susceptibility-related effects in a tissue matrix. Phantom study. Magn Reson Med 1998; 39: 417–428.
- 17 Young IR, Cox IJ, Bryant DJ, Bydder GM. The benefits of increasing spatial resolution as a means of reducing artifacts due to field inhomogeneities. Magn Reson Imag 1988; 6: 585–590.
- 18
Haacke EM,
Tkach JA,
Parrish TB.
Reduction of T
 dephasing in gradient field-echo imaging.
Radiology
1989;
170:
457–462.
dephasing in gradient field-echo imaging.
Radiology
1989;
170:
457–462.
- 19 Cho ZH, Ro YM. Reduction of susceptibility artifact in gradient-echo imaging. Magn Reson Med 1992; 23: 193–200.
- 20 Frahm J, Merboldt KD, Hanicke W. Direct FLASH MR imaging of magnetic field inhomogeneities by gradient compensation. Magn Reson Med 1988; 6: 474–480.
- 21 Yang QX, Dardzinski BJ, Li S, Eslinger PJ, Smith MB. Multi-gradient echo with susceptibility inhomogeneity compensation (MGESIC): demonstration of fMRI in the olfactory cortex at 3.0 T. Magn Reson Med 1997; 37: 331–335.
- 22
Constable RT,
Spencer DD.
Composite image formation in z-shimmed functional MR imaging.
Magn Reson Med
1999;
42:
110–117.
10.1002/(SICI)1522-2594(199907)42:1<110::AID-MRM15>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 23
Glover GH.
3D z-shim method for reduction of susceptibility effects in BOLD fMRI.
Magn Reson Med
1999;
42:
290–299.
10.1002/(SICI)1522-2594(199908)42:2<290::AID-MRM11>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 24 Fernandez-Seara MA, Wehrli FW. Postprocessing technique to correct for background gradients in image-based R2* measurements. Magn Reson Med 2000; 44: 358–366.
- 25
Springer C.
Physicochemical principles influencing magnetopharmaceuticals, NMR in physiology and biomedicine.
San Diego:
Academic Press;
1994. p
75–99.
10.1016/B978-0-12-283980-1.50010-7 Google Scholar
- 26 Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 1994; 32: 749–763.
- 27 Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med 1992; 24: 375–383.
- 28 Hardy PA, Kucharczyk W, Henkelman RM. Cause of signal loss in MR images of old hemorrhagic lesions. Radiology 1990; 174: 549–555.
- 29 Yablonskiy DA, Reinus WR, Stark H, Haacke EM. Quantitation of T2' anisotropic effects on magnetic resonance bone mineral density measurement. Magn Reson Med 1997; 37: 214–221.
- 30 Eichling JO, Raichle ME, Grubb RL Jr, Larson KB, Ter-Pogossian MM. In vivo determination of cerebral blood volume with radioactive oxygen-15 in the monkey. Circ Res 1975; 37: 707–714.
- 31 Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Stoddart HF. Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg 1996; 82: 269–277.
- 32 Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Widman R. The influence of carbon dioxide and body position on near- infrared spectroscopic assessment of cerebral hemoglobin oxygen saturation. Anesth Analg 1996; 82: 278–287.
- 33
Duong TQ,
Kim SG.
In vivo MR measurements of regional arterial and venous blood volume fractions in intact rat brain.
Magn Reson Med
2000;
43:
393–402.
10.1002/(SICI)1522-2594(200003)43:3<393::AID-MRM11>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 34 Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 1994; 31: 9–21.
- 35 Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 1995; 34: 555–566.
- 36
Kiselev VG,
Posse S.
Analytical model of susceptibility-induced MR signal dephasing: effect of diffusion in a microvascular network.
Magn Reson Med
1999;
41:
499–509.
10.1002/(SICI)1522-2594(199903)41:3<499::AID-MRM12>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar