Rational Design, Synthesis and Evaluation of Coumarin Derivatives as Protein-protein Interaction Inhibitors
Corresponding Author
Laura De Luca
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorCorresponding Author
Fatima E. Agharbaoui
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorRosaria Gitto
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorMaria Rosa Buemi
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorFrauke Christ
Molecular Virology and Gene Therapy KU Leuven and IRC KULAK, Kapucijnenvoer 33, B-3000 Leuven, Flanders, Belgium
Search for more papers by this authorZeger Debyser
Molecular Virology and Gene Therapy KU Leuven and IRC KULAK, Kapucijnenvoer 33, B-3000 Leuven, Flanders, Belgium
Search for more papers by this authorStefania Ferro
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorCorresponding Author
Laura De Luca
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorCorresponding Author
Fatima E. Agharbaoui
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorRosaria Gitto
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorMaria Rosa Buemi
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorFrauke Christ
Molecular Virology and Gene Therapy KU Leuven and IRC KULAK, Kapucijnenvoer 33, B-3000 Leuven, Flanders, Belgium
Search for more papers by this authorZeger Debyser
Molecular Virology and Gene Therapy KU Leuven and IRC KULAK, Kapucijnenvoer 33, B-3000 Leuven, Flanders, Belgium
Search for more papers by this authorStefania Ferro
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università degli Studi di Messina, Viale Annunziata, I-98168 Messina, Italy
Search for more papers by this authorGraphical Abstract
Abstract
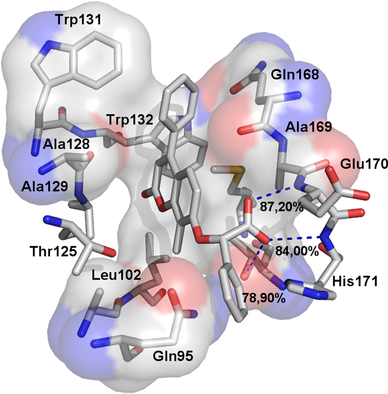
Herein we describe the design and synthesis of a new series of coumarin derivatives searching for novel HIV-1 integrase (IN) allosteric inhibitors. All new obtained compounds were tested in order to evaluate their ability to inhibit the interaction between the HIV-1 IN enzyme and the nuclear protein lens epithelium growth factor LEDGF/p75. A combined approach of docking and molecular dynamic simulations has been applied to clarify the activity of the new compounds. Specifically, the binding free energies by using the method of molecular mechanics-generalized Born surface area (MM-GBSA) was calculated, whereas hydrogen bond occupancies were monitored throughout simulations methods.
References
- 1UNAIDS 2015. Global AIDS Response Progress Reporting 2015.
- 2D. Zhao, K. Lu, J. Mol. Model. 2015, 21, 299.
- 3E. De Clercq, Int. J. Antimicrob. Agents 2009, 33, 307–320.
- 4Y. Mehellou, E. De Clercq, J. Med. Chem. 2010, 53, 521–538.
- 5T. Juday, S. Gupta, K. Grimm, S. Wagner, E. Kim, HIV Clin. Trials 2011, 12, 71–78.
- 6A. Ammassari, M. P. Trotta, N. Shalev, P. Marconi, A. Antinori, Antivir. Ther. 2012, 17, 785–792.
- 7P. Ryscavage, S. Kelly, J. Z. Li, P. R. Harrigan, B. Taiwo, Antimicrob. Agents. Chemother. 2014, 58, 3585–3598.
- 8S. A. Riddler, R. Haubrich, A. G. DiRienzo, L. Peeples, W. G. Powderly, K. L. Klingman, K. W. Garren, T. George, J. F. Rooney, B. Brizz, U. G. Lalloo, R. L. Murphy, S. Swindells, D. Havlir, J. W. Mellors, N. Engl. J. Med. 2008, 358, 2095–2106.
- 9S. A. Bozzette, C. F. Ake, H. K. Tam, S. W. Chang, T. A. Louis, N. Engl. J. Med. 2003, 348, 702–710.
- 10P. W. Hruz, Curr. Opin. HIV AIDS 2008, 3, 660–665.
- 11L. De Luca, S. Ferro, F. Morreale, A. Chimirri, Mini Rev. Med. Chem. 2011, 11, 714–727.
- 12L. De Luca, S. Ferro, F. Morreale, S. De Grazia, A. Chimirri, ChemMedChem 2011, 6, 1184–1191.
- 13J. Demeulemeester, P. Chaltin, A. Marchand, M. De Maeyer, Z. Debyser, F. Christ, Expert. Opin. Ther. Pat. 2014, 24, 609–632.
- 14F. Christ, Z. Debyser, Virology 2013, 435, 102–109.
- 15S. Hare, F. Di Nunzio, A. Labeja, J. Wang, A. Engelman, P. Cherepanov, PLoS Pathog. 2009, 5, e 1000515.
- 16G. Maertens, P. Cherepanov, W. Pluymers, K. Busschots, E. De Clercq, Z. Debyser, Y. Engelborghs, J. Biol. Chem. 2003, 278, 33528–33539.
- 17M. Llano, M. Vanegas, O. Fregoso, D. Saenz, S. Chung, M. Peretz, E. M. Poeschla, J. Virol. 2004, 78, 9524–9537.
- 18K. Busschots, A. Voet, M. De Maeyer, J. C. Rain, S. Emiliani, R. Benarous, L. Desender, Z. Debyser, F. Christ, J. Mol. Biol. 2007, 365, 1480–1492.
- 19S. Rahman, R. Lu, N. Vandegraaff, P. Cherepanov, A. Engelman, Virology 2007, 357, 79–90.
- 20S. Emiliani, A. Mousnier, K. Busschots, M. Maroun, B. Van Maele, D. Tempe, L. Vandekerckhove, F. Moisant, L. Ben-Slama, M. Witvrouw, F. Christ, J. C. Rain, C. Dargemont, Z. Debyser, R. Benarous, J. Biol. Chem. 2005, 280, 25517–25523.
- 21M. C. Shun, J. E. Daigle, N. Vandegraaff, A. Engelman, J. Virol. 2007, 81, 166–172.
- 22H. M. Marshall, K. Ronen, C. Berry, M. Llano, H. Sutherland, D. Saenz, W. Bickmore, E. Poeschla, F. D. Bushman, PLoS One 2007, 2, e 1340.
- 23A. Hombrouck, J. De Rijck, J. Hendrix, L. Vandekerckhove, A. Voet, M. De Maeyer, M. Witvrouw, Y. Engelborghs, F. Christ, R. Gijsbers, Z. Debyser, PLoS Pathog. 2007, 3, e 47.
- 24F. Christ, S. Shaw, J. Demeulemeester, B. A. Desimmie, A. Marchand, S. Butler, W. Smets, P. Chaltin, M. Westby, Z. Debyser, C. Pickford, Antimicrob. Agents Chemother. 2012, 56, 4365–4374.
- 25F. Christ, A. Voet, A. Marchand, S. Nicolet, B. A. Desimmie, D. Marchand, D. Bardiot, N. J. Van der Veken, B. Van Remoortel, S. V. Strelkov, M. De Maeyer, P. Chaltin, Z. Debyser, Nat .Chem. Biol. 2010, 6, 442–448.
- 26J. J. Kessl, N. Jena, Y. Koh, H. Taskent-Sezgin, A. Slaughter, L. Feng, S. de Silva, L. Wu, S. F. Le Grice, A. Engelman, J. R. Fuchs, M. Kvaratskhelia, J. Biol .Chem. 2012, 287, 16801–16811.
- 27L. De Luca, F. Morreale, F. Christ, Z. Debyser, S. Ferro, R. Gitto, Eur. J. Med. Chem. 2013, 68, 405–411.
- 28L. De Luca, S. Ferro, F. Morreale, A. Chimirri, Mini-Reviews in Medicinal Chemistry 2011, 11, 714–727.
- 29A. Sharma, A. Slaughter, N. Jena, L. Feng, J. J. Kessl, H. J. Fadel, N. Malani, F. Male, L. Wu, E. Poeschla, F. D. Bushman, J. R. Fuchs, M. Kvaratskhelia, PLoS Pathog. 2014, 10, e 1004171.
- 30D. J. Newman, G. M. Cragg, J. Nat. Prod. 2012, 75, 311–335.
- 31Y. W. Chin, M. J. Balunas, H. B. Chai, A. D. Kinghorn, AAPS J. 2006, 8, E 239–253.
- 32S. Robert, C. Bertolla, B. Masereel, J. M. Dogne, L. Pochet, J. Med. Chem. 2008, 51, 3077–3080.
- 33R. Frederick, S. Robert, C. Charlier, J. de Ruyck, J. Wouters, B. Pirotte, B. Masereel, L. Pochet, J. Med. Chem. 2005, 48, 7592–7603.
- 34T. Taechowisan, C. Lu, Y. Shen, S. Lumyong, J Cancer Res Ther 2007, 3, 86–91.
- 35M. Itoigawa, C. Ito, H. T. Tan, M. Kuchide, H. Tokuda, H. Nishino, H. Furukawa, Cancer Lett 2001, 169, 15–19.
- 36V. Reutrakul, P. Leewanich, P. Tuchinda, M. Pohmakotr, T. Jaipetch, S. Sophasan, T. Santisuk, Planta Med. 2003, 69, 1048–1051.
- 37C. Spino, M. Dodier, S. Sotheeswaran, Bioorg. Med. Chem. Lett. 1998, 8, 3475–3478.
- 38H. A. Stefani, K. Gueogjan, F. Manarin, S. H. Farsky, J. Zukerman-Schpector, I. Caracelli, S. R. Pizano Rodrigues, M. N. Muscara, S. A. Teixeira, J. R. Santin, I. D. Machado, S. M. Bolonheis, R. Curi, M. A. Vinolo, Eur. J. Med. Chem. 2012, 58, 117–127.
- 39L. De Luca, M. L. Barreca, S. Ferro, F. Christ, N. Iraci, R. Gitto, A. M. Monforte, Z. Debyser, A. Chimirri, ChemMedChem 2009, 4, 1311–1316.
- 40L. De Luca, S. Ferro, R. Gitto, M. L. Barreca, S. Agnello, F. Christ, Z. Debyser, A. Chimirri, Bioorg. Med. Chem. 2010, 18, 7515–7521.
- 41L. De Luca, R. Gitto, F. Christ, S. Ferro, S. De Grazia, F. Morreale, Z. Debyser, A. Chimirri, Antiviral. Res. 2011, 92, 102–107.
- 42L. De Luca, F. Morreale, A. Chimirri, J. Chem. Inf. Model. 2012, 52, 3245–3254.
- 43S. Ferro, L. De Luca, G. Lo Surdo, F. Morreale, F. Christ, Z. Debyser, R. Gitto, A. Chimirri, Bioorg. Med. Chem. 2014, 22, 2269–2279.
- 44S. Ferro, L. De Luca, F. Morreale, F. Christ, Z. Debyser, R. Gitto, A. Chimirri, J. Enzyme Inhib. Med. Chem. 2014, 29, 237–242.
- 45S. Ferro, L. De Luca, F. E. Agharbaoui, F. Christ, Z. Debyser, R. Gitto, Bioorg. Med. Chem. 2015, 23, 3208–3214.
- 46L. Q. Al-Mawsawi, F. Christ, R. Dayam, Z. Debyser, N. Neamati, FEBS Lett. 2008, 582, 1425–1430.
- 47P. Cherepanov, A. L. Ambrosio, S. Rahman, T. Ellenberger, A. Engelman, Proc. Natl. Acad. Sci. USA 2005, 102, 17308–17313.
- 48The PyMOL Molecular Graphics System, DeLano Scientific LLC.
- 49R. A. Laskowski, M. B. Swindells, J .Chem. Inf. Model. 2011, 51, 2778–2786.
- 50G. Jones, P. Willett, R. C. Glen, A. R. Leach, R. Taylor, J. Mol. Biol. 1997, 267, 727–748.
- 51P. A. Kollman, I. Massova, C. Reyes, B. Kuhn, S. Huo, L. Chong, M. Lee, T. Lee, Y. Duan, W. Wang, O. Donini, P. Cieplak, J. Srinivasan, D. A. Case, T. E. Cheatham, 3rd, Acc. Chem. Res. 2000, 33, 889–897.
- 52D. A. Darden, T. A. Case, T. E. Cheatham, III, C. L. Simmerling, J. Wang, R. E. Duke, R. Luo, R. C. Walker, W. Zhang, K. M. Merz, B. Roberts, B. Wang, S. Hayik, A. Roitberg, G. Seabra, I. Kolossváry, K. F. Wong, F. Paesani, J. Vanicek, J. Liu, X. Wu, S. R. Brozell, T. Steinbrecher, H. Gohlke, Q. Cai, X. Ye, J. Wang, M.-J. Hsieh, G. Cui, D. R. Roe; D. H. Mathews, M. G. Seetin, C. Sagui, V. Babin, T. Luchko, S. Gusarov, A. Kovalenko, P. A. Kollman, Amber 11. San Francisco: University of California 2010.
- 53R. Pauwels, J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, E. De Clercq, J. Virol. Methods 1988, 20, 309–321.
- 54Accelrys, Catalyst: http://www.accelrys.com.
- 55S. Maignan, J. P. Guilloteau, Q. Zhou-Liu, C. Clement-Mella, V. Mikol, J. Mol. Biol. 1998, 282, 359–368.
- 56Maestro, Schrödinger, LLC, New York, NY.
- 57Y. Duan, C. Wu, S. Chowdhury, M. C. Lee, G. Xiong, W. Zhang, R. Yang, P. Cieplak, R. Luo, T. Lee, J. Caldwell, J. Wang, P. Kollman, J. Comput. Chem. 2003, 24, 1999–2012.
- 58J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman, D. A. Case, J. Comput. Chem. 2004, 25, 1157–1174.