Ring-Opening Homo- and Copolymerization of Cis/Trans-3-oxa-4-oxobicyclo- and Cis/Trans-4-oxa-3-oxobicyclo[5.4.0]undecane
Abstract
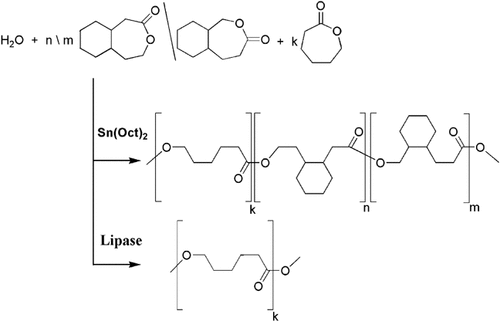
Ring-opening polymerization of the bicyclic lactone mixture 2, cis/trans-3-oxa-4-oxo- and cis/trans-4-oxa-3-oxobicyclo[5.4.0]undecane with Sn(Oct)2 as a catalyst was investigated for the first time (Scheme 1). The lactones were obtained by Baeyer–Villiger oxidation of cis/trans-2-decalone 1 (Scheme 2). Additionally, copolymerization of 2 with ε-caprolactone in different ratios was performed. GPC measurements and 1H NMR spectroscopy proved that 2 was quantitatively incorporated into the polymer, leading to polycaprolactone with cyclohexane moieties in its backbone. 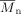 values were up to 25 000 with
values were up to 25 000 with 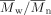 ≤ 2.4. DSC measurements revealed a linear dependence of the melting points and the glass transition temperatures of the polymers on the feed of the bicyclic lactone 2. Additionally, the time dependence of the cis/trans ratio of the residual monomers was followed during the course of polymerization reaction by gas chromatography. The crystallinity of the resulting copolymers was investigated via polarization microscopy. Finally, it was shown that the mixture of 2 with ε-caprolactone could be selectively polymerized with lipase. Only ε-caprolactone was converted, while 2 remained unreacted in the residue.
≤ 2.4. DSC measurements revealed a linear dependence of the melting points and the glass transition temperatures of the polymers on the feed of the bicyclic lactone 2. Additionally, the time dependence of the cis/trans ratio of the residual monomers was followed during the course of polymerization reaction by gas chromatography. The crystallinity of the resulting copolymers was investigated via polarization microscopy. Finally, it was shown that the mixture of 2 with ε-caprolactone could be selectively polymerized with lipase. Only ε-caprolactone was converted, while 2 remained unreacted in the residue.