Saccharide Polymers 6
Abstract
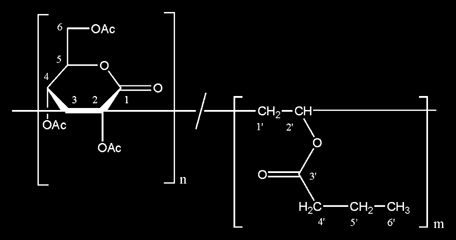
Summary: The 2,4,6-tri-O-acetyl-3-deoxy-D-erythro-hex-2-enono-1,5-lactone, briefly Ac-Gluc-enlactone (GEL) (1), is easily synthesized in an one-step reaction from glucono-δ-lactone with good yields. Free radical polymerizations of GEL with vinyl esters with side chain of different length including fatty acids esters were carried out in substance or in solution under various conditions. The structures and chemical compositions of the polymers were established by elemental analysis, FT-IR-, 1H-, and 13C NMR spectroscopy. Copolymerization kinetics were investigated. Characteristic properties of polymers, e.g. molecular weight, optical rotation, and thermal properties are reported. The products are of interest as components in commodities with binding and adhesive properties.