Dynamic characteristics of
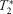 -weighted signal in calf muscles of peripheral artery disease during low-intensity exercise
-weighted signal in calf muscles of peripheral artery disease during low-intensity exercise
Zhijun Li MD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
Search for more papers by this authorMatthew D. Muller PhD
Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorJianli Wang MD, PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorChristopher T. Sica PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorPrasanna Karunanayaka PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorLawrence I. Sinoway MD
Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorCorresponding Author
Qing X. Yang PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Address reprint requests to: Q.X.Y., Department of Radiology, Penn State University College of Medicine, 500 University Dr., Hershey, PA 17033. E-mail: [email protected]Search for more papers by this authorZhijun Li MD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
Search for more papers by this authorMatthew D. Muller PhD
Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorJianli Wang MD, PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorChristopher T. Sica PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorPrasanna Karunanayaka PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorLawrence I. Sinoway MD
Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Search for more papers by this authorCorresponding Author
Qing X. Yang PhD
Department of Radiology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA
Address reprint requests to: Q.X.Y., Department of Radiology, Penn State University College of Medicine, 500 University Dr., Hershey, PA 17033. E-mail: [email protected]Search for more papers by this authorAbstract
Purpose
To evaluate the dynamic characteristics of
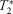 -weighted signal change in exercising skeletal muscle of healthy subjects and peripheral artery disease (PAD) patients under a low-intensity exercise paradigm.
-weighted signal change in exercising skeletal muscle of healthy subjects and peripheral artery disease (PAD) patients under a low-intensity exercise paradigm.
Materials and Methods
Nine PAD patients and nine age- and sex-matched healthy volunteers underwent a low-intensity exercise paradigm while magnetic resonance imaging (MRI) (3.0T) was obtained.
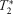 -weighted signal time-courses in lateral gastrocnemius, medial gastrocnemius, soleus, and tibialis anterior were acquired and analyzed. Correlations were performed between dynamic
-weighted signal time-courses in lateral gastrocnemius, medial gastrocnemius, soleus, and tibialis anterior were acquired and analyzed. Correlations were performed between dynamic
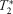 -weighted signal and changes in heart rate, mean arterial pressure, leg pain, and perceived exertion.
-weighted signal and changes in heart rate, mean arterial pressure, leg pain, and perceived exertion.
Results
A significant signal decrease was observed during exercise in soleus and tibialis anterior of healthy participants (P = 0.0007–0.04 and 0.001–0.009, respectively). In PAD, negative signals were observed (P = 0.008–0.02 and 0.003–0.01, respectively) in soleus and lateral gastrocnemius during the early exercise stage. Then the signal gradually increased above the baseline in the lateral gastrocnemius during and after exercise in six of the eight patients who completed the study. This signal increase in patients' lateral gastrocnemius was significantly greater than in healthy subjects' during the later exercise stage (two-sample t-tests, P = 0.001–0.03). Heart rate and mean arterial pressure responses to exercise were significantly higher in PAD than healthy subjects (P = 0.036 and 0.008, respectively) and the patients experienced greater leg pain and exertion (P = 0.006 and P = 0.0014, respectively).
Conclusion
During low-intensity exercise, there were different dynamic
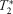 -weighted signal behavior in the healthy and PAD exercising muscles.
-weighted signal behavior in the healthy and PAD exercising muscles.
Level of Evidence: 2
Technical Efficacy: Stage 1
J. MAGN. RESON. IMAGING 2017;46:40–48
References
- 1 Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc NatI Acad Sci USA 1990; 87: 9868–9872.
- 2 Jacobi B, Bongartz G, Partovi S, et al. Skeletal muscle BOLD MRI: from underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging 2012; 35: 1253–1265.
- 3 Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 1998; 162: 411–419.
- 4 Wagner PD. Muscle intracellular oxygenation during exercise: optimization for oxygen transport, metabolism, and adaptive change. Eur J Appl Physiol 2011; 112: 1–8.
- 5 Mortensen SP, Svendsen JH, Ersbøll M, et al. Skeletal muscle signaling and the heart rate and blood pressure response to exercise: insight from heart rate pacing during exercise with a trained and a deconditioned muscle group. Hypertension 2013; 61: 1126–1133.
- 6 Duling BR, Klitzman B. Local control of microvascular function: role in tissue oxygen supply. Annu Rev Physiol 1980; 42: 373–382.
- 7 Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340.
- 8 Hirsch AU, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463–654.
- 9 Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381–386.
- 10 Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation 1990; 82: 1925–1931.
- 11 Bondke Persson A, Buschmann EE, Lindhorst R, et al. Therapeutic arteriogenesis in peripheral arterial disease: combining intervention and passive training. Vasa 2011; 40: 177–187.
- 12 Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med 2015; 45: 231–244.
- 13 Aherne T, McHugh S, Kheirelseid EA, et al. Comparing supervised exercise therapy to invasive measures in the management of symptomatic peripheral arterial disease. Surg Res Pract 2015; 2015: 960402.
- 14 West AM, Anderson JD, Epstein FH, et al. Percutaneous intervention in peripheral artery disease improves calf muscle phosphocreatine recovery kinetics: a pilot study. Vasc Med 2012; 17: 3–9.
- 15 Bajwa A, Wesolowski R, Patel A, et al. Blood oxygenation level-dependent CMR-derived measures in critical limb ischemia and changes with revascularization. J Am Coll Cardiol 2016; 67: 420–431.
- 16 Stacy MR, Caracciolo CM, Qiu M, et al. Comparison of regional skeletal muscle tissue oxygenation in college athletes and sedentary control subjects using quantitative BOLD MR imaging. Physiol Rep 2016;4:pii: e12903.
- 17 Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): An update for practitioners. Vasc Health Risk Manag 2009; 5: 833–841.
- 18 Muller MD, Li Z, Sica CT, et al. Muscle oxygenation during dynamic plantar flexion exercise: combining BOLD MRI with traditional physiological measurements. Physiol Rep 2016; 4:pii: e13004.
- 19 Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381.
- 20 Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 2013; 64: 240–256.
- 21 Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by nearinfrared spectroscopy. Eur J Vasc Surg 1994; 8: 294–296.
- 22 Komiyama T, Onozuka A, Miyata T, Shigematsu H. Oxygen saturation measurement of calf muscle during exercise in intermittent claudication. Eur J Vasc Endovasc Surg 2002; 23: 388–392.
- 23 Osada T, Katsumura T, Murase N, et al. Post-exercise hyperemia after ischemic and non-ischemic isometric handgrip exercise. J Physiol Anthropol Appl Human Sci 2003; 22: 299–309.
- 24 Villar R, Hughson RL. Repeatability of popliteal blood flow and lower limb vascular conductance at rest and exercise during body tilt using Doppler ultrasound. Physiol Meas 2013; 34: 291–306.
- 25 Hiatt WR, Marsh RC, Brammell HL, Fee C, Horwitz LD. Effect of aerobic conditioning on the peripheral circulation during chronic beta-adrenergic blockade. J Am Coll Cardiol 1984; 4: 958–963.
- 26 Granger HJ. Goodman AH, Cook BH. Metabolic models of microcirculatory regulation. Fed Proc 1975; 34: 2025–2030.
- 27 Haddy FJ, Scott JB. Metabolic factors in peripheral circulatory regulation. Fed Proc 1975; 34: 2006–2011.
- 28 Honig CR. Contributions of nerves and metabolites to exercise vasodilation: a unifying hypothesis. Am J Physiol 1979; 236: H705–719.
- 29 Johnson PC, Henrich HA. Metabolic and myogenic factors in local regulation of the microcirculation. Fed Proc 1975; 34: 2020–2024.
- 30 Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Uğurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med 1995; 33: 453–459.
- 31 Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage 2004; 23(Suppl 1): S220–233.
- 32 Cain SM, Chapler CK. O2 extraction by canine hindlimb during alpha-adrenergic blockade and hypoxic hypoxia. J Appl Physiol Respir Environ Exerc Physiol 1980; 48: 630–635.
- 33 Schaffartzik W, Barton ED, Poole DC, et al. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J Appl Physiol (1985) 1993; 75: 491–498.
- 34 King-Vanvlack CE, Curtis SE, Mewburn JD, Cain SM, Chapler CK. Role of endothelial factors in active hyperemic responses in contracting canine muscle. J Appl Physiol (1985) 1995; 79: 107–112.
- 35 Frahm J, Merboldt KD, Hänicke W, Kleinschmidt A, Boecker H. Brain or vein—oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR Biomed 1994; 7: 45–53.
- 36 Zheng Y, Martindale J, Johnston D, Jones M, Berwick J, Mayhew J. A model of the hemodynamic response and oxygen delivery to brain. Neuroimage 2002; 16(3 Pt 1): 617–637.
- 37 Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med 1997; 24: 308–320.
- 38 Schmid AI, Schewzow K, Fiedler GB, et al. Exercising calf muscle T2* changes correlate with pH, PCr recovery and maximum oxidative phosphorylation. NMR Biomed 2014; 27: 553–560.
- 39 Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease. Part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 2007-2008; 41: 481–489.
- 40 Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease. Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg 2008; 42: 101–112.
- 41 Brass EP, Wang H, Hiatt WR. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc Med 2000; 5: 225–230.
- 42 Englund EK, Langham MC, Li C, et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson 2013; 15: 70.
- 43 Englund EK, Langham MC, Ratcliffe SJ, et al. Multiparametric assessment of vascular function in peripheral artery disease: dynamic measurement of skeletal muscle perfusion, blood-oxygen-level dependent signal, and venous oxygen saturation. Circ Cardiovasc Imaging 2015; 8:pii: e002673.
- 44 Isbell DC, Berr SS, Toledano AY, et al. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol 2006; 47: 2289–2295.
- 45 Thompson RB, Aviles RJ, Faranesh AZ, et al. Measurement of skeletal muscle perfusion during postischemic reactive hyperemia using contrast-enhanced MRI with a step-input function. Magn Reson Med 2005; 54: 289–298.
- 46 Ledermann HP, Schulte AC, Heidecker HG, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation 2006; 113: 2929–2935.
- 47 Schewzow K, Andreas M, Moser E, Wolzt M, Schmid AI. Automatic model-based analysis of skeletal muscle BOLD-MRI in reactive hyperemia. J Magn Reson Imaging 2013; 38: 963–969.
- 48 Schewzow K, Fiedler GB, Meyerspeer M, et al. Dynamic ASL and T2-weighted MRI in exercising calf muscle at 7 T: a feasibility study. Magn Reson Med 2015; 73: 1190–1195.
- 49 Celis R, Pipinos II, Scott-Pandorf MM, et al. Peripheral arterial disease affects kinematics during walking. J Vasc Surg 2009; 49: 127–132.




