Blunted fat oxidation upon submaximal exercise is partially compensated by enhanced glucose metabolism in children, adolescents, and young adults with Barth syndrome
Corresponding Author
William Todd Cade
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Correspondence
William T. Cade, Washington University School of Medicine, Campus Box 8502, 4444 Forest Park Avenue, St. Louis, MO 63108.
Email: [email protected]
Search for more papers by this authorKathryn L. Bohnert
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorLinda R. Peterson
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorBruce W. Patterson
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdam J. Bittel
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdewole L. Okunade
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorLisa de las Fuentes
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorKaren Steger-May
Division of Biostatistics, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdil Bashir
Department of Radiology, Washington University School of Medicine, St. Louis, Missouri
Department of Electrical and Computer Engineering, Auburn University, Auburn, Alabama
Search for more papers by this authorGeorge G. Schweitzer
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorShaji K. Chacko
Department of Pediatrics, Baylor College of Medicine, Houston, Texas
Search for more papers by this authorRonald J. Wanders
Department of Pediatrics, University of Amsterdam, Amsterdam, The Netherlands
Search for more papers by this authorChristina A. Pacak
Department of Pediatrics, University of Florida, Gainesville, Florida
Search for more papers by this authorBarry J. Byrne
Department of Pediatrics, University of Florida, Gainesville, Florida
Search for more papers by this authorDominic N. Reeds
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorCorresponding Author
William Todd Cade
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Correspondence
William T. Cade, Washington University School of Medicine, Campus Box 8502, 4444 Forest Park Avenue, St. Louis, MO 63108.
Email: [email protected]
Search for more papers by this authorKathryn L. Bohnert
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorLinda R. Peterson
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorBruce W. Patterson
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdam J. Bittel
Program in Physical Therapy, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdewole L. Okunade
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorLisa de las Fuentes
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorKaren Steger-May
Division of Biostatistics, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAdil Bashir
Department of Radiology, Washington University School of Medicine, St. Louis, Missouri
Department of Electrical and Computer Engineering, Auburn University, Auburn, Alabama
Search for more papers by this authorGeorge G. Schweitzer
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorShaji K. Chacko
Department of Pediatrics, Baylor College of Medicine, Houston, Texas
Search for more papers by this authorRonald J. Wanders
Department of Pediatrics, University of Amsterdam, Amsterdam, The Netherlands
Search for more papers by this authorChristina A. Pacak
Department of Pediatrics, University of Florida, Gainesville, Florida
Search for more papers by this authorBarry J. Byrne
Department of Pediatrics, University of Florida, Gainesville, Florida
Search for more papers by this authorDominic N. Reeds
Department of Medicine, Washington University School of Medicine, St. Louis, Missouri
Search for more papers by this authorAbstract
Barth syndrome (BTHS) is a rare X-linked condition resulting in abnormal mitochondria, cardioskeletal myopathy, and growth delay; however, the effects of BTHS on substrate metabolism regulation and their relationships with tissue function in humans are unknown. We sought to characterize glucose and fat metabolism during rest, submaximal exercise, and postexercise rest in children, adolescents, and young adults with BTHS and unaffected controls and examine their relationships with cardioskeletal energetics and function. Children/adolescents and young adults with BTHS (n = 29) and children/adolescent and young adult control participants (n = 28, total n = 57) underwent an infusion of 6′6′H2 glucose and U-13C palmitate and indirect calorimetry during rest, 30-minutes of moderate exercise (50% 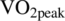 ), and recovery. Cardiac function, cardioskeletal mitochondrial energetics, and exercise capacity were examined via echocardiography, 31P magnetic resonance spectroscopy, and peak exercise testing, respectively. The glucose turnover rate was significantly higher in individuals with BTHS during rest (33.2 ± 9.8 vs 27.2 ± 8.1 μmol/kgFFM/min, P < .01) and exercise (34.7 ± 11.2 vs 29.5 ± 8.8 μmol/kgFFM/min, P < .05) and tended to be higher postexercise (33.7 ± 10.2 vs 28.8 ± 8.0 μmol/kgFFM/min, P < .06) compared to controls. Increases in total fat (−3.9 ± 7.5 vs 10.5 ± 8.4 μmol/kgFFM/min, P < .0001) and plasma fatty acid oxidation rates (0.0 ± 1.8 vs 5.1 ± 3.9 μmol/kgFFM/min, P < .0001) from rest to exercise were severely blunted in BTHS compared to controls. Conclusion: An inability to upregulate fat metabolism during moderate intensity exercise appears to be partially compensated by elevations in glucose metabolism. Derangements in fat and glucose metabolism are characteristic of the pathophysiology of BTHS.
), and recovery. Cardiac function, cardioskeletal mitochondrial energetics, and exercise capacity were examined via echocardiography, 31P magnetic resonance spectroscopy, and peak exercise testing, respectively. The glucose turnover rate was significantly higher in individuals with BTHS during rest (33.2 ± 9.8 vs 27.2 ± 8.1 μmol/kgFFM/min, P < .01) and exercise (34.7 ± 11.2 vs 29.5 ± 8.8 μmol/kgFFM/min, P < .05) and tended to be higher postexercise (33.7 ± 10.2 vs 28.8 ± 8.0 μmol/kgFFM/min, P < .06) compared to controls. Increases in total fat (−3.9 ± 7.5 vs 10.5 ± 8.4 μmol/kgFFM/min, P < .0001) and plasma fatty acid oxidation rates (0.0 ± 1.8 vs 5.1 ± 3.9 μmol/kgFFM/min, P < .0001) from rest to exercise were severely blunted in BTHS compared to controls. Conclusion: An inability to upregulate fat metabolism during moderate intensity exercise appears to be partially compensated by elevations in glucose metabolism. Derangements in fat and glucose metabolism are characteristic of the pathophysiology of BTHS.
A severely blunted ability to upregulate fat metabolism during a modest level of physical activity is a defining pathophysiologic characteristic in children, adolescents, and young adults with BTHS.
CONFLICT OF INTEREST
W.T.C. declares, K.L.B., L.R.P., B.W.P., A.J.B., A.L.O., L.d.l.F., K.S.-M., A.B., G.G.S., S.K.C., R.J.W., C.A.P., B.J.B., and D.N.R. declare that they have no conflict of interest.
Supporting Information
| Filename | Description |
|---|---|
| jimd12094-sup-0001-TableS1.docxWord 2007 document , 22.7 KB | Table S1. Substrate metabolism during rest, exercise, and postexercise in children |
| jimd12094-sup-0002-TableS2.docxWord 2007 document , 24.2 KB | Table S2. Substrate metabolism during rest, exercise, and postexercise in adults |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Barth PG, Scholte HR, Berden JA, et al. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. 1983; 62: 327-355.
- 2Clarke SL, Bowron A, Gonzalez IL, et al. Barth syndrome. Orphanet J Rare Dis. 2013; 8: 23.
- 3Barth PG, Valianpour F, Bowen VM, et al. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A. 2004; 126A: 349-354.
- 4Bione S, D'Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D. A novel X-linked gene, G4.5. Is responsible for Barth syndrome. Nat Genet. 1996; 12: 385-389.
- 5Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014; 20: 616-623.
- 6Xu Y, Phoon CK, Berno B, et al. Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat Chem Biol. 2016; 12: 641-647.
- 7Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002; 277: 43553-43556.
- 8Bashir A, Bohnert KL, Reeds DN, et al. Impaired cardiac and skeletal muscle bioenergetics in children, adolescents, and young adults with Barth syndrome. Physiol Rep. 2017;Feb; 5(3). pii: e13130.
- 9Cade WT, Spencer CT, Reeds DN, et al. Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis. 2013; 36: 91-101.
- 10Houten SM, Violante S, Ventura FV, Wanders RJ. The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu Rev Physiol. 2016; 78: 23-44.
- 11Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993; 265: E380-E391.
- 12van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol. 2003; 553: 611-625.
- 13Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18: 1440-1463.
- 14Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991; 40: 406-409.
- 15Jeppesen TD, Orngreen MC, van Hall G, Haller RG, Vissing J. Fat metabolism during exercise in patients with mitochondrial disease. Arch Neurol. 2009; 66: 365-370.
- 16Orngreen MC, Jeppesen TD, Andersen ST, et al. Fat metabolism during exercise in patients with McArdle disease. Neurology. 2009; 72: 718-724.
- 17Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol. 1995; 269: E649-E656.
- 18Reeds DN, Yarasheski KE, Fontana L, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006; 290: E47-E53.
- 19Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999; 40: 2118-2124.
- 20Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959; 82: 420-430.
- 21Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983; 55: 628-634.
- 22Kiens B, Lithell H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J Clin Invest. 1989; 83: 558-564.
- 23Orngreen MC, Duno M, Ejstrup R, et al. Fuel utilization in subjects with carnitine palmitoyltransferase 2 gene mutations. Ann Neurol. 2005; 57: 60-66.
- 24Orngreen MC, Norgaard MG, Sacchetti M, van Engelen BG, Vissing J. Fuel utilization in patients with very long-chain acyl-coa dehydrogenase deficiency. Ann Neurol. 2004; 56: 279-283.
- 25Laforet P, Orngreen M, Preisler N, Andersen G, Vissing J. Blocked muscle fat oxidation during exercise in neutral lipid storage disease. Arch Neurol. 2012; 69: 530-533.
- 26Riley M, Elborn JS, Bell N, Stanford CF, Nicholls DP. Substrate utilization during exercise in chronic cardiac failure. Clin Sci (Lond). 1990; 79: 89-95.
- 27Wang Y, Mohsen AW, Mihalik SJ, Goetzman ES, Vockley J. Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J Biol Chem. 2010; 285: 29834-29841.
- 28Suzuki-Hatano S, Saha M, Rizzo SA, et al. AAV-mediated TAZ gene replacement restores mitochondrial and cardioskeletal function in Barth syndrome. Hum Gene Ther. 2019; 30: 139-154.
- 29Knottnerus SJG, Bleeker JC, Wust RCI, et al. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord. 2018; 19: 93-106.
- 30Gaspard GJ, McMaster CR. Cardiolipin metabolism and its causal role in the etiology of the inherited cardiomyopathy Barth syndrome. Chem Phys Lipids. 2015; 193: 1-10.
- 31Acehan D, Vaz F, Houtkooper RH, et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011; 286: 899-908.
- 32McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol. 2006; 361: 462-469.
- 33Spencer CT, Byrne BJ, Bryant RM, et al. Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol. 2011; 301: H2122-H2129.
- 34Klein S, Coyle EF, Wolfe RR. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Physiol. 1994; 267: E934-E940.
- 35Schweitzer GG, Finck BN, Cade WT. Increased anaerobic metabolism during exercise in Barth syndrome may result from augmented liver glycogenolysis (abstract). Paper presented at: Barth Syndrome Foundation Scientific, Medical & Family Conference; 2018; Clearwater Beach, FL.
- 36Vissing J, Galbo H, Haller RG. Exercise fuel mobilization in mitochondrial myopathy: a metabolic dilemma. Ann Neurol. 1996; 40: 655-662.
- 37Jeppesen TD, Orngreen MC, Van Hall G, Vissing J. Lactate metabolism during exercise in patients with mitochondrial myopathy. Neuromuscul Disord. 2013; 23: 629-636.
- 38Jensen MD, Cryer PE, Johnson CM, Murray MJ. Effects of epinephrine on regional free fatty acid and energy metabolism in men and women. Am J Physiol. 1996; 270: E259-E264.
- 39Coderre L, Srivastava AK, Chiasson JL. Role of glucocorticoid in the regulation of glycogen metabolism in skeletal muscle. Am J Physiol. 1991; 260: E927-E932.
- 40Kolnes AJ, Birk JB, Eilertsen E, Stuenaes JT, Wojtaszewski JF, Jensen J. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles. Am J Physiol Endocrinol Metab. 2015; 308: E231-E240.
- 41Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010; 26: 615-629.
- 42Jacob S, Rett K, Wicklmayr M, Agrawal B, Augustin HJ, Dietze GJ. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. J Hypertens. 1996; 14: 489-494.
- 43Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004; 292: 2227-2236.
- 44Celik T, Iyisoy A, Kursaklioglu H, et al. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J Hypertens. 2006; 24: 591-596.




