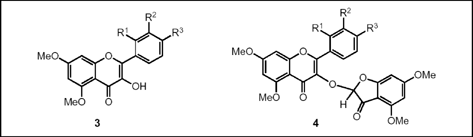
Darzens reaction of 2-bromo-4,6-dimethoxy-3(2H)-benzofuranone with aromatic aldehydes to form flavonoids
Philipp A. Ottersbach
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorDavid Bolek
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorEva Lepičová
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorMichael Gütschow
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorMartin Nieger
Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, PO Box 55, FIN-00014 University of Helsinki, Finland
Search for more papers by this authorPhilipp A. Ottersbach
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorDavid Bolek
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorEva Lepičová
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorMichael Gütschow
Pharmaceutical Institute, Pharmaceutical Chemistry I, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany
Search for more papers by this authorMartin Nieger
Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, PO Box 55, FIN-00014 University of Helsinki, Finland
Search for more papers by this authorAbstract
The applicability of 2-bromo-4,6-dimethoxy-3(2H)-benzofuranone (1) to produce flavonoid-derived epoxides in the course of the Darzens reaction with aldehydes was investigated. However, instead of the epoxides, flavonols 3 and, in certain cases, benzofuranyl-substituted flavonols 4 were isolated. The generation of 3 is assumed due to a ring expansion of the initially formed epoxides. These flavonols can react with 1 to produce the unexpected 1:2 adducts 4 as minor products. The structure of the hexamethoxy derivative 4b (R1 = H, R2 = R3 = OMe) was confirmed by X-ray crystallographic analysis.
References and Notes
- 1a Harborne, J. B.; Williams, C. A. Phytochemistry, 2000, 55, 481. 1b Boumendjel, A. Curr. Med. Chem., 2003, 10, 2621.
- 2a Alcaráz, L. E.; Blanco S. E.; Puig, O. N.; Tomás, R.; Ferretti, F. H. J. Theor. Biol., 2000, 205, 231. 2b Krauze-Baranowska, M.; Wiwart, M. Z. Naturforsch. [c], 2003, 58, 65.
- 3 Narender, T.; Khaliq, T.; Shweta; Nishi; Goyal, N.; Gupta, S. Bioorg. Med. Chem., 2005, 13, 6543.
- 4 Chiang, L. C.; Chiang, W.; Liu, M. C.; Lin, C. C. J. Antimicrob. Chemother., 2003, 58, 65.
- 5a Ruiz, P. A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. J. Nutr., 2007, 137, 1208. 5b Read, M. A. Am. J. Pathol., 1995, 147, 235.
- 6 Cárdenas, M.; Marder, M.; Blank, V. C.; Roguin, L. P. Bioorg. Med. Chem., 2006, 14, 2966.
- 7a Crazier, A.; Burns, J.; Aziz, A. A.; Stewart, A. J.; Rabiasz, H. S.; Jenkins, G. I.; Edwards, C. A.; Lean, M. E. Biol. Res., 2000, 33, 79. 7b Westenburg, H. E.; Lee, K. J.; Lee, S. K.; Fong, H. S.; van Breemen, R. B.; Pezzuto, J. M., Kinghorn, A. D. J. Nat. Prod., 2000, 63, 1696.
- 8a Hadjeri, M.; Barbier, M.; Ronot, X.; Mariotte, A. M.; Boumendjel, A.; Boutonnat, J. J. Med. Chem., 2003, 46, 2125. 8b Boumendjel, A.; Beney, C.; Deka, N.; Mariotte, A. M.; Lawson, M. A.; Trompier, D.; Baubichon-Cortay, H.; Di Retro, A. Chem. Pharm. Bull., 2002, 50, 854.
- 9 Braune, A.; Gütschow, M.; Engst, W.; Blaut, M. Appl. Environ. Microbiol., 2001, 67, 5558.
- 10 Löser, R.; Chlupacova, M.; Marecek, A.; Opletalova, V.; Gutschow, M. Helv. Chim. Acta., 2004, 87, 2597.
- 11 Brady, B. A.; Geoghegan, M.; McMurtrey, K. D.; O'Sullivan, W. J. Chem. Soc., Perkin Trans. I, 1981, 119.
- 12 Bolek, D.; Gütschow, M. J. Heterocyclic Chem., 2005, 42, 1399.
- 13 Adam, W.; Curci, R.; Edwards, J. O. Acc. Chem. Res., 1989, 22, 205.
- 14
Rosen, T.,
In Comprehensive Organic Synthesis,
B. M. Trost;
I. Fleming Ed,
Pergamon Press, Oxford,
1991,
Vol 2,
pp 409–439.
10.1016/B978-0-08-052349-1.00035-4 Google Scholar
- 15 Bennett, M.; Burke, A. J.; O'Sullivan, W. I. Tetrahedron, 1996, 52, 7163.
- 16 Beney, C.; Mariotte, A. M.; Boumendjel, A. Heterocycles, 2001, 55, 967.
- 17 Jacques, J.; Marquet, A. Org. Syntheses, 1988, Coll. Vol. 6, 175.
- 18 Pelter, A.; Ward, R. S.; Hänsel, R.; Khaliefi, F. J. Chem. Soc., Perkin Trans I, 1981, 3182.
- 19 McGarry, L. W.; Detty, M. R. J. Org. Chem., 1990, 55, 4349.
- 20 De la Torre, M. D.; Rodrigues, A. G.; Tomé, A. C.; Siva, A. M.; Cavaleiro, J. A. Tetrahedron, 2004, 60, 3581.
- 21 Sheldrick, G. M. Acta Cryst. 1990, A46, 467.
- 22 Sheldrick, G. M. SHELXL97. University of Göttingen, Germany 1997.
- 23 Sheldrick, G. M. SHELXTL. Version 5. Bruker AXS Inc., Madison Wisconsin, USA 2001.
- 24 Brandenburg, K. DIAMOND. Version 3.1d. Crystal Impact GbR, Bonn, Germany 2006.