Synthesis and biological activities of novel 1,3-bis[substituted-pyridyl (thiazolyl)methyl]-2-substitutedmethylideneimidazolidines
Abstract
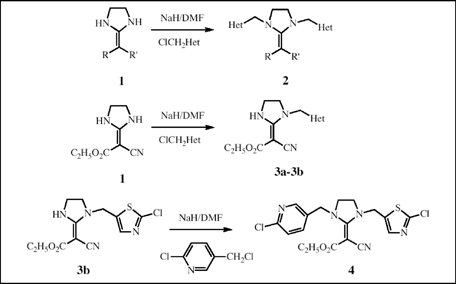
A series of novel 1,3-dissubstitutedpyridyl(thiazolyl)methyl-2-substituted-methylideneimidazolidine derivatives 2 and 4 were designed and synthesized via the N-alkylation of the disubstituted heterocyclic ketene aminal derivative 1. When 1 (R = CN, R' = COOC2H5) was used as the starting materials, mono N-alkylated reaction can take place in good yields owing to the presence of the intramolecular hydrogen bond. However, as for 1 (R = R' = CN), it is difficult to obtain pure mono N-alkylated product. The structures of the target compounds were confirmed by IR, 1H NMR, EI-MS and elemental analyses, and, in the case of 2c, by single crystal X-ray diffraction. The preliminary bioassay indicated that some of the title compounds possess moderate fungicidal and insecticidal activity.