New therapeutic targets in ulcerative colitis: The importance of ion transporters in the human colon
Klaudia Farkas MD
First Department of Medicine, University of Szeged, Szeged, Hungary
The first two authors contributed equally.
Search for more papers by this authorSunil Yeruva MSc, PhD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
The first two authors contributed equally.
Search for more papers by this authorZoltán Rakonczay Jr MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorLisa Ludolph MSc
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorTamás Molnár MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorFerenc Nagy MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorZoltán Szepes MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorAndrea Schnúr MSc
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorTibor Wittmann MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorJessica Hubricht MD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorBrigitte Riederer MSc, PhD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorViktória Venglovecz MSc, PhD
Department of Pharmacology and Pharmacotherapy, University of Szeged, Szeged, Hungary
Search for more papers by this authorGyörgy Lázár MD, PhD
Department of Surgery, University of Szeged, Szeged, Hungary
Search for more papers by this authorMarianna Király MSc
Department of Oral Biology, Semmelweis University, Budapest, Hungary
Search for more papers by this authorÁkos Zsembery MD, PhD
Institute of Human Physiology and Clinical Experimental Research, Semmelweis University, Budapest, Hungary
Search for more papers by this authorGábor Varga MSc, PhD, DSc
Department of Oral Biology, Semmelweis University, Budapest, Hungary
Search for more papers by this authorUrsula Seidler XX
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorCorresponding Author
Péter Hegyi MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
First Department of Medicine, University of Szeged, H-6720, Korányi fasor 8-10, Szeged, HungarySearch for more papers by this authorKlaudia Farkas MD
First Department of Medicine, University of Szeged, Szeged, Hungary
The first two authors contributed equally.
Search for more papers by this authorSunil Yeruva MSc, PhD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
The first two authors contributed equally.
Search for more papers by this authorZoltán Rakonczay Jr MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorLisa Ludolph MSc
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorTamás Molnár MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorFerenc Nagy MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorZoltán Szepes MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorAndrea Schnúr MSc
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorTibor Wittmann MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
Search for more papers by this authorJessica Hubricht MD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorBrigitte Riederer MSc, PhD
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorViktória Venglovecz MSc, PhD
Department of Pharmacology and Pharmacotherapy, University of Szeged, Szeged, Hungary
Search for more papers by this authorGyörgy Lázár MD, PhD
Department of Surgery, University of Szeged, Szeged, Hungary
Search for more papers by this authorMarianna Király MSc
Department of Oral Biology, Semmelweis University, Budapest, Hungary
Search for more papers by this authorÁkos Zsembery MD, PhD
Institute of Human Physiology and Clinical Experimental Research, Semmelweis University, Budapest, Hungary
Search for more papers by this authorGábor Varga MSc, PhD, DSc
Department of Oral Biology, Semmelweis University, Budapest, Hungary
Search for more papers by this authorUrsula Seidler XX
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover, Germany
Search for more papers by this authorCorresponding Author
Péter Hegyi MD, PhD
First Department of Medicine, University of Szeged, Szeged, Hungary
First Department of Medicine, University of Szeged, H-6720, Korányi fasor 8-10, Szeged, HungarySearch for more papers by this authorAbstract
Background:
The absorption of water and ions (especially Na+ and Cl−) is an important function of colonic epithelial cells in both physiological and pathophysiological conditions. Despite the comprehensive animal studies, there are only scarce available data on the ion transporter activities of the normal and inflamed human colon.
Methods:
In this study, 128 healthy controls and 69 patients suffering from ulcerative colitis (UC) were involved. We investigated the expressional and functional characteristics of the Na+/H+ exchangers (NHE) 1–3, the epithelial sodium channel (ENaC), and the SLC26A3 Cl−/HCO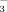 exchanger downregulated in adenoma (DRA) in primary colonic crypts isolated from human biopsy and surgical samples using microfluorometry, patch clamp, and real-time reverse-transcription polymerase chain reaction (RT-PCR) techniques.
exchanger downregulated in adenoma (DRA) in primary colonic crypts isolated from human biopsy and surgical samples using microfluorometry, patch clamp, and real-time reverse-transcription polymerase chain reaction (RT-PCR) techniques.
Results:
Data collected from colonic crypts showed that the activities of electroneutral (via NHE3) and the electrogenic Na+ absorption (via ENaC) are in inverse ratio to each other in the proximal and distal colon. We found no significant differences in the activity of NHE2 in different segments of the colon. Surface cell Cl−/HCO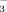 exchange is more active in the distal part of the colon. Importantly, both sodium and chloride absorptions are damaged in UC, whereas NHE1, which has been shown to promote immune response, is upregulated by 6-fold.
exchange is more active in the distal part of the colon. Importantly, both sodium and chloride absorptions are damaged in UC, whereas NHE1, which has been shown to promote immune response, is upregulated by 6-fold.
Conclusions:
These results open up new therapeutic targets in UC. (Inflamm Bowel Dis 2011;)
REFERENCES
- 1 Becker JM, Stucchi AF. Treatment of choice for acute severe steroid-refractory ulcerative colitis is colectomy. Inflamm Bowel Dis. 2009; 15: 146–149.
- 2 Németh HZ, Deitch EA, Szabó C, et al. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol. 2002; 283: 122–132.
- 3 Laubitz D, Larmonier CB, Bai A, et al. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008; 295: G63–G77.
- 4 Seidler U, Lenzen H, Cinar A, et al. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann N Y Acad Sci. 2006; 1072: 262–275.
- 5 Teleky B, Hamilton G, Cosentini E, et al. Intracellular pH regulation of human colonic crypts cells. Pflugers Arch. 1994; 426: 267–275.
- 6 Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanism and implications for disease. Physiol Rev. 2002; 82: 245–289.
- 7 Musch M, Arvans DL, WU GD, et al. Functional coupling of the downregulated in adenoma Cl-/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol. 2009; 296: G202–210.
- 8 Clauss W, Schäfer H, Horch I, et al. Segmental differences in electrical properties and Na-transport of rabbit cecum, proximal and distal colon in vitro. Pflugers Arch. 1985; 403: 278–282.
- 9 Yau WM, Makhlouf GM. Comparison of transport mechanisms in isolated ascending and descending rat colon. Am J Physiol. 1975; 228: 191–195.
- 10 Ikuma M, Kahgarian M, Binder HJ, et al. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol Gastrointest Liver Physiol. 1999; 276: G539–G549.
- 11 Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol. 2010 [Epub ahead of print].
- 12 Levitan R, Fordtran JS, Burrows BA, et al. Water and salt absorption in the human colon. J Clin Invest. 1962; 41: 1754–1759.
- 13 Sandle GI, Wills NK, Alles W, et al. Electrophysiology of the human colon: evidence of segmental heterogeneity. Gut. 1986; 27: 999–1005.
- 14 Kawamata K, Hayashi H, Suzuki Y. Chloride dependent bicarbonate secretion in the mouse large intestine. Biomed Res. 2006; 27: 15–21.
- 15 Roediger WE, Lawson MJ, Kwok V, et al. Colonic bicarbonate output as a test of disease activity in ulcerative colitis. J Clin Pathol. 1984; 37: 704–707.
- 16 Schilli R, Breuer RI, Klein F, et al. Comparison of the composition of faecal fluid in Crohn's disease and ulcerative colitis. Gut. 1982; 23: 326–332.
- 17 Weijers HA, Van De Kamer JH. Alteration of intestinal bacterial flora as a cause of diarrhoea. Nutr Abstr Rev. 1965; 35: 591–604.
- 18 Sullivan S, Alex P, Dassopoulos T, et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: Potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis. 2009; 15: 261–274.
- 19 Greig ER, Boot-Handford RP, Mani V, et al. Decreased expression of apical Na+ channels and basolateral Na+-K+-ATPase in ulcerative colitis. J Pathol. 2004; 204: 84–92.
- 20 Civitelli R, Teitelbaum SL, Hruska KA, et al. IL-1 activates the Na+/H+ antiport in a murine T cell. J Immunol. 1989; 143: 4000–4008.
- 21 Vairo G, Royston AK, Hamilton JA. Biochemical events accompanying macrophage activation and the inhibition of colony-stimulating factor-1-induced macrophage proliferation by tumor necrosis factor-α, interferon-γ, and lipopolysaccharide. J Cell Physiol. 1992; 151: 630–641.
- 22 Prpic V, Yu SF, Figueiredo F, et al. Role of Na+/H+ exchange by interferon-gamma in enhanced expression of JE and I-A beta genes. Science. 1989; 244: 469–471.
- 23 Orlinska U, Newton RC. Modification of tumor necrosis factor-alpha (TNF-α) production by the Na+ dependent HCO3-cotransport in lipopolysaccharide-activated human monocytes. Immunopharmacology. 1995; 30: 41–50.
- 24 Thomas JA, Buchsbaum RN, Zimniak A, et al. Intracellular pH-measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979; 18: 2210–2218.
- 25 Hegyi P, Rakonczay Z Jr, Gray MA, et al. Measurement of intracellular pH in pancreatic duct cells: a new method for calibrating the fluorescence data. Pancreas. 2004; 28: 427–434.
- 26 Bachmann O, Riederer B, Rossmann H, et al. The Na+/H+ exchanger isoform 2 is the predominant NHE isoform in murine colonic crypts and its lack causes NHE3 upregulation. Am J Physiol Gastrointest Liver Physiol. 2004; 287: G125–133.
- 27 Cinar A, Chen M, Riederer B, et al. NHE3 inhibition by cAMP and Ca2+ is abolished in PDZ-domain protein PDZK1-deficient murine enterocytes. J Physiol. 2007; 581: 1235–1246.
- 28 Kiela PR, Ghishan FK. Na+-H+ exchange in mammalian digestive tract. In: LR Johnson, ed. Physiology of the Gastrointestinal Tract. Amsterdam: Elsevier Press; 2006: 1847–1881.
- 29 Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005; 67: 411–443.
- 30 Broere N, Chen M, Cinar, et al. Defective jejunal and colonic salt absorption and altered Na+/H+ exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflugers Arch. 2009; 457: 1079–1091.
- 31 Scholz W, Albus U, Counillon L, et al. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995; 29: 260–268.
- 32 Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004; 1: 4–9.
- 33 Nesterov V, Dahlmann A, Bertog M, et al. Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nepron. Am J Physiol Renal Physiol. 2008; 295: F1052–F1062.
- 34 Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009; 284: 20447–20451.
- 35 Kellenberger S, Gautschi I, Schild L. Mutations in the epithelial Na+ channel ENaC outer pore disrupt amiloride block by increasing its dissociation rate. Mol Pharmacol. 2003; 64: 848–856.
- 36 Jacob P, Rossmann H, Lamprecht G, et al. Down-regulated in adenoma mediates apical Cl-/HCO3- exchange in rabbit, rat, and human duodenum. Gastroenterology. 2002; 22: 709–724.
- 37 Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: LR Johnson, ed. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994: 2133–2171.
- 38 Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998; 43: 294–299.
- 39 Sellin JH, De Soignie R. Ion transport in human colon in vitro. Gastroenterology. 1987; 93: 441–448.
- 40 Devroede GJ, Phillips SF. Failure of the human rectum to absorb electrolytes and water. Gut. 1970; 11: 438–442.
- 41 Devroede GJ, Phillips SF, Code CF, et al. Regional differences in rates of insorption of sodium and water from the human large intestine. Can J Physiol Pharmacol. 1971; 49: 1023–1029.
- 42 Cho JH, Musch MW, Bookstein CM, et al. Aldosterone stimulates intestinal Na+ absorption in rats by increasing NHE3 expression of the proximal colon. Am J Physiol. 1998; 274: C586–C594.
- 43 Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998; 19: 282–285.
- 44 Ledoussal C, Woo AL, Miller ML, et al. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001; 281: G1385–G1396.
- 45 Hasselblatt P, Warth R, Schulz-Baldes A, et al. pH regulation in isolated in vitro perfused rat colonic crypts. Pflugers Arch. 2000; 441: 118–124.
- 46 Wang Z, Wang T, Petrovic S, et al. Renal and intestinal transport defect in Slc26a6-null mice. Am J Physiol Cell Physiol. 2004; 288: C957–C965.
- 47 Schweinfest CW, Spyropoulos DD, Henderson KW, et al. Slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006; 281: 37962–37971.
- 48 Mäkelä S, Kere J, Holmberg C, et al. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002; 20: 425–438.
- 49 Ikuma M, Geibel J, Binder HJ, et al. Characterization of Cl-HCO3 exchange in basolateral membrane of rat distal colon. Am J Physiol Cell Physiol. 2003; 285: C912–C921.
- 50 Rajendran VM, Binder HJ. Characterization and molecular localization of anion transporters in colonic epithelial cells. Ann N Y Acad Sci. 2000; 915: 15–29.
- 51 De Vito P. The sodium/hydrogen exchanger: a possible mediator of immunity. Cell Immunol. 2006; 240: 69–85.
- 52 Ohmori Y, Reynolds E, Hamilton TA. Modulation of Na+/K+ exchange potentiates lipopolysaccharide-induced gene expression in murine peritoneal macrophages. J Cell Physiol. 1991; 148: 96–105.
- 53 Rosengren S, Henson PM, Worthen GS. Migration associated volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol Cell Physiol. 1994; 267: C1623–C1632.
- 54 Witko-Sarsat V, Allen RC, Paulais M, et al. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol. 1996; 157: 2728–2735.
- 55 Amasheh S, Barmeyer C, Koch CS, et al. Cytokine dependent transcriptional down-regulation of epithelial down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004; 126: 1711–1720.
- 56 Yeruva S, Farkas K, Hubricht J, et al. Preserved Na+/H+ exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis. 2010; 16: 1149–1161.
- 57 He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol. 2010; 2010: 238080.
- 58 Yang H, Jiang W, Furth EE, et al. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol. 1998; 275: G1445–1453.
- 59 McKenzie SJ, Baker MS, Buffinton GD, et al. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996; 98: 136–141.
- 60 Saksena S, Gill RK, Tyagi S, et al. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl-/OH- exchange activity in human intestinal epithelial cells. Biochem J. 2008; 416: 99–108.




