Correlating Boron–Hydrogen Stretching Frequencies with Boron–Hydrogen Bond Lengths in Closoboranes: An Approach Using DFT Calculations
Hans Hagemann
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorManish Sharma
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorDaniel Sethio
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorLatévi Max Lawson Daku
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorHans Hagemann
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorManish Sharma
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorDaniel Sethio
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorLatévi Max Lawson Daku
Dépt. de Chimie Physique, Université de Genève, 30, quai E. Ansermet, CH-1211 Geneva 4, Switzerland
Search for more papers by this authorAbstract
Using harmonic and anharmonic DFT calculations, we have established a general correlation between B–H stretching frequencies and B–H bond lengths valid for the closoboranes 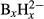 (x = 6 – 12), substituted closoboranes B12H12 –
(x = 6 – 12), substituted closoboranes B12H12 – 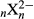 (with X = F, Cl, Br and n = 1 – 3 and 9 – 12) and the carboranes
(with X = F, Cl, Br and n = 1 – 3 and 9 – 12) and the carboranes 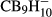 and
and 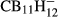 , suggesting that this correlation is also applicable to other similar species. It appears that the average B–H stretching frequency observed around 2500 cm−1 shift by about −100 cm−1 if the average B–H bond length increases by 1 pm. In contrast to
, suggesting that this correlation is also applicable to other similar species. It appears that the average B–H stretching frequency observed around 2500 cm−1 shift by about −100 cm−1 if the average B–H bond length increases by 1 pm. In contrast to 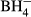 , the B–H bond in closoboranes is practically covalent and the correlation evidenced between its stretching frequency and its length proves to be similar to the one observed for the C–H bond.
, the B–H bond in closoboranes is practically covalent and the correlation evidenced between its stretching frequency and its length proves to be similar to the one observed for the C–H bond.
References
- 1L. Schlapbach, A. Züttel, ‘Hydrogen-Storage Materials for Mobile Applications’, Nature 2001, 414, 353 – 358.
- 2S. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel, C. M. Jensen, ‘Complex Hydrides for Hydrogen Storage’, Chem. Rev. 2007, 107, 4111 – 4132.
- 3M. B. Ley, L. H. Jepsen, Y.-S. Lee, Y. W. Cho, J. M. Bellosta von Colbe, M. Dornheim, M. Rokni, J. O. Jensen, M. Sloth, Y. Filinchuk, J. E. Jørgensen, F. Besenbacher, T. R. Jensen, ‘Complex Hydrides for Hydrogen Storage – New Perspectives’, Mater. Today 2014, 17, 122 – 128.
- 4R. Mohtadi, S. Orimo, ‘The Renaissance of Hydrides as Energy Materials’, Nat. Rev. Mater. 2016, 2, 16091.
- 5W. S. Tang, M. Matsuo, H. Wu, V. Stavila, W. Zhou, A. A. Talin, A. V. Soloninin, R. V. Skoryunov, O. A. Babanova, A. V. Skripov, A. Unemoto, S. Orimo, T. J. Udovic, ‘Liquid-Like Ionic Conduction in Solid Lithium and Sodium Monocarba-closo-Decaborates Near or at Room Temperature’, Adv. Energy Mater. 2016, 6, 1502237.
- 6N. Verdal, J.-H. Her, V. Stavila, A. V. Soloninin, O. A. Babanova, A. V. Skripov, T. J. Udovic, J. J. Rush, ‘Complex High-Temperature Phase Transitions in Li2B12H12 and Na2B12H12’, J. Solid State Chem. 2014, 212, 81 – 91.
- 7N. Verdal, H. Wu, T. J. Udovic, V. Stavila, W. Zhou, J. J. Rush, ‘Evidence of a Transition to Reorientational Disorder in the Cubic Alkali-Metal Dodecahydro-closo-dodecaborates’, J. Solid State Chem. 2011, 184, 3110 – 3116.
10.1016/j.jssc.2011.09.010 Google Scholar
- 8K. Yoshida, T. Sato, A. Unemoto, M. Matsuo, T. Ikeshoji, T. J. Udovic, S. Orimo, ‘Fast Sodium Ionic Conduction in Na2B10H10-Na2B12H12 Pseudo-Binary Complex Hydride and Application to a Bulk-Type All-Solid-State Battery’, Appl. Phys. Lett. 2017, 110, 103901.
- 9L. Duchêne, R.-S. Kühnel, D. Rentsch, A. Remhof, H. Hagemann, C. Battaglia, ‘A Highly Stable Sodium Solid-State Electrolyte Based on a Dodeca/Deca-Borate Equimolar Mixture’, Chem. Commun. 2017, 53, 4195 – 4198.
- 10Y. Sadikin, M. Brighi, P. Schouwink, R. Černý, ‘Superionic Conduction of Sodium and Lithium in Anion-Mixed Hydroborates Na3BH4B12H12 and (Li0.7Na0.3)3BH4B12H12’, Adv. Energy Mater. 2015, 5, 1501016.
- 11Y. Sadikin, P. Schouwink, M. Brighi, Z. Łodziana, R. Černý, ‘Modified Anion Packing of Na2B12H12 in Close to Room Temperature Superionic Conductors’, Inorg. Chem. 2017, 56, 5006 – 5016.
- 12R. M. Badger, ‘A Relation Between Internuclear Distances and Bond Force Constants’, J. Chem. Phys. 1934, 2, 128 – 131.
- 13H. J. Bernstein, ‘The Average XH Stretching Frequency as a Measure of XH Bond Properties’, Spectrochim. Acta 1962, 18, 161 – 170.
- 14D. C. McKean, ‘Non-Equivalent CH Bonds in CH3 Compounds, from CD2H Infrared Studies’, Chem. Commun. 1971, 1373 – 1375.
- 15D. C. McKean, ‘Individual CH Bond Strengths in Simple Organic Compounds: Effects of Conformation and Substitution’, Chem. Soc. Rev. 1978, 7, 399 – 422.
- 16R. G. Snyder, A. L. Aljibury, H. L. Strauss, H. L. Casal, K. M. Gough, W. F. Murphy, ‘Isolated C-H Stretching Vibrations of n-Alkanes: Assignments and Relation to Structure’, J. Chem. Phys. 1984, 81, 5352 – 5361.
- 17V. D'Anna, L. M. Lawson Daku, H. Hagemann, ‘Quantitative Spectra–Structure Relations for Borohydrides’, J. Phys. Chem. C 2015, 119, 21868 – 21874.
10.1021/acs.jpcc.5b06045 Google Scholar
- 18P. Carbonnière, H. Hagemann, ‘Fermi Resonances of Borohydrides in a Crystalline Environment of Alkali Metals’, J. Phys. Chem. A 2006, 110, 9927 – 9933.
- 19M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian, Inc., Wallingford, CT, USA, 2009.
- 20A. D. Becke, ‘Density-Functional Thermochemistry. III. The Role of Exact Exchange’, J. Chem. Phys. 1993, 98, 5648 – 5652.
- 21P. C. Hariharan, J. A. Pople, ‘The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies’, Theoretica Chim. Acta 1973, 28, 213 – 222.
- 22‘ Gaussian NEWS, V. 5, no. 2, summer 1994, p. 2. “Becke3LYP Method References and General Citation Guidelines”’.
- 23D. Sethio, L. M. Lawson Daku, H. Hagemann, ‘Computational Study of the Vibrational Spectroscopy Properties of Boron-Hydrogen Compounds: Mg(B3H8)2, CB9H10− and CB11H12−, Int. J. Hydrogen Energy 2017, 42, 22496 – 22501.
- 24M. Sharma, D. Sethio, V. D'Anna, H. Hagemann, ‘Theoretical Study of B12HnF(12 – n)2− Species’, Int. J. Hydrogen Energy 2015, 40, 12721 – 12726.
- 25M. Sharma, H. Hagemann, manuscript in preparation.
- 26V. Barone, ‘Vibrational Zero-Point Energies and Thermodynamic Functions Beyond the Harmonic Approximation’, J. Chem. Phys. 2004, 120, 3059 – 3065.
- 27V. Barone, ‘Anharmonic Vibrational Properties by a Fully Automated Second-Order Perturbative Approach’, J. Chem. Phys. 2005, 122, 014108.
- 28J. Bloino, V. Barone, ‘A Second-Order Perturbation Theory Route to Vibrational Averages and Transition Properties of Molecules: General Formulation and Application to Infrared and Vibrational Circular Dichroism Spectroscopies’, J. Chem. Phys. 2012, 136, 124108.
- 29S. Grimme, ‘Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction’, J. Comput. Chem. 2006, 27, 1787 – 1799.
- 30R. Caputo, S. Garroni, D. Olid, F. Teixidor, S. Suriñach, M. D. Baró, ‘Can Na2[B12H12] be a Decomposition Product of NaBH4?’, Phys. Chem. Chem. Phys. 2010, 12, 15093 – 15100.
- 31T. H. Dunning Jr., ‘Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron Through Neon and Hydrogen’, J. Chem. Phys. 1989, 90, 1007 – 1023.
- 32L. He, H.-W. Li, H. Nakajima, N. Tumanov, Y. Filinchuk, S.-J. Hwang, M. Sharma, H. Hagemann, E. Akiba, ‘Synthesis of a Bimetallic Dodecaborate LiNaB12H12 with Outstanding Superionic Conductivity’, Chem. Mater. 2015, 27, 5483 – 5486.
- 33D. Sethio, L. M. Lawson Daku, H. Hagemann, ‘A Theoretical Study of the Spectroscopic Properties of B2H6 and of a Series of Bx Species (x = 1–12, y = 3–14, z = 0–2): From BH3 to B12H122−, Int. J. Hydrogen Energy 2016, 41, 6814 – 6824.
- 34V. Benham, G. Lord, I. S. Butler, D. F. R. Gilson, ‘High-Pressure Diamond-Anvil-Cell Micro-Raman Spectra of Mercuric Cyanide, Hg(CN)2, and Cesium Dodecahydroborate, Cs2[B12H12]’, Appl. Spectrosc. 1987, 41, 915 – 917.
- 35Y. Filinchuk, A. V. Talyzin, H. Hagemann, V. Dmitriev, D. Chernyshov, B. Sundqvist, ‘Cation Size and Anion Anisotropy in Structural Chemistry of Metal Borohydrides. The Peculiar Pressure Evolution of RbBH4’, Inorg. Chem. 2010, 49, 5285 – 5292.




