Transition-Metal-Free, Visible-Light-Driven Regioselective Phosphinoylation/Hydrazonation of Unactivated Alkenes with Arylhydrazines and H-Phosphine Oxides
Weifeng Xu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Minjing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLongzhi Zhu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorSha Jin
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Biquan Xiong
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
E-mail: [email protected]; [email protected]Search for more papers by this authorWeifeng Xu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Minjing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLongzhi Zhu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorSha Jin
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Biquan Xiong
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
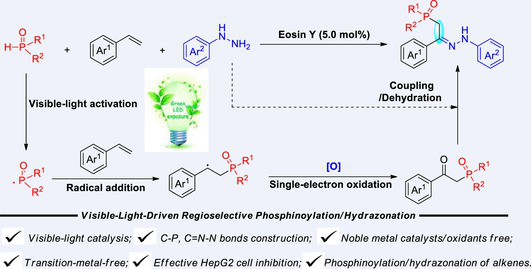
A novel visible-light-driven protocol has been established for the direct difunctionalization of unactivated alkenes using arylhydrazines and H-phosphine oxides as dual-function reagents. Through visible-light photocatalysis, phosphonyl radicals are generated as key intermediates, which undergo a cascade process involving radical addition, single-electron oxidation, and dehydration coupling to achieve the in-situ construction of C–P and C=N–N bonds. The method demonstrates broad substrate compatibility with excellent functional group tolerance, delivering β-phosphinoyl hydrazones in moderate to good yields. Notably, several synthesized compounds exhibit potent anti-proliferative activity against HepG2 cells. Mechanistic investigations through radical trapping experiments and kinetic studies confirm a radical chain pathway, with photocatalysis crucially mediating the initial radical generation and subsequent electron transfer processes.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500527-sup-0001-supinfo.pdfPDF document, 7.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Rollas, S.; Güniz Küçükgüzel, Ş. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939; (b) Xiao, L. X.; Li, K.; Meng, L. A convenient synthesis of novel 7-phosphonylbenzyl-2-substituted pyrazolo [4,3-e]-1,2,4-triazolo [1,5-c]-pyrimidine derivatives. Heteroat. Chem. 2008, 19, 634–638; (c) Alberca, S.; Romero Parra, J.; Fernández López, I. Enantioselective synthesis of α-aryl α-hydrazino phosphonates. Chem. Sci. 2024, 15, 7725; (d) Wang, Y.; Guo, S.; Yu, L. Hydrazone derivatives in agrochemical discovery and development. Chin. Chem. Lett. 2024, 35, 108207; (e) Kölmel, D. K.; Kool, E. T.; Oximes and hydrazones in bioconjugation: mechanism and catalysis. Chem. Rev. 2017, 117, 10358–10376; (f) Verma, G.; Marella, A.; Shaquiquzzaman, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80.
- 2(a) Goulioukina, N. S.; Shergold, I. A.; Rybakov, V. B. One-Pot Two-Step Synthesis of Optically Active α-Amino Phosphonates by Palladium-Catalyzed Hydrogenation/Hydrogenolysis of α-Hydrazono Phosphonates. Adv. Synth. Catal. 2017, 359, 153–162; (b) Mao, S.; Gao, Y. R.; Zhu, X. Q.; Copper-catalyzed radical reaction of N-tosylhydrazones: Stereoselective synthesis of (E)-vinyl sulfones. Org. Lett. 2015, 17, 1692–1695; (c) Yan, K.; He, H.; Li, J. Blue light-promoted cyclopropenizations of N-tosylhydrazones in water. Chin. Chem. Lett. 2021, 32, 3984–3987; (d) Zhang, X.; Ning, Y.; Liu, Z. Defluorinative carboimination of trifluoromethyl ketones. ACS Catal. 2022, 12, 8802–8810; (e) Li, B. L.; Li, S.; Zhang, C. Photoclick and Release for Spatiotemporally Localized Theranostics of Single Cells via In Situ Generation of 1,3-Diaryl-1H-benzo[f]indazole-4,9-dione. Angew. Chem. Int. Ed. 2025, 64, e202416111; (f) Jin, J.; Lv, Y.; Tang, W. Enantioselective Transformation of Hydrazones via Remote NHC Catalysis: Activation Across C=N and N-N Bonds. ACS Catal. 2024, 14, 18378–18384; (g) Yang, Y.; Sivaguru, P.; Song, Q. Copper-Catalyzed Cyclopropenation of Alkynes with Difluoromethyl Carbene. Chin. J. Chem. 2025, 43, 1833–1840; (h) Zhang, Z.; Zhou, Q.; Yu, W. Cu (I)-Catalyzed Three-Component Coupling of Trifluoromethyl Ketone N-Tosylhydrazones, Alkynes and Azides: Synthesis of Difluoromethylene Substituted 1,2,3-Triazoles. Chin. J. Chem. 2017, 35, 387–391; (i) Sivaguru, P.; Pan, Y.; Wang, N. Who is Who in the Carbene Chemistry of N-Sulfonyl Hydrazones. Chin. J. Chem. 2024, 42, 2071–2108.
- 3(a) Chew, S. T.; Lo, K. M.; Lee, S. K. Copper complexes with phosphonium containing hydrazone ligand: Topoisomerase inhibition and cytotoxicity study. Eur. J. Med. Chem. 2014, 76, 397–407; (b) Murugesan, A.; Mani, S. K.; Koochakkhani, S. Design, synthesis and anticancer evaluation of novel arylhydrazones of active methylene compounds. Int. J. Biol. Macromol. 2024, 254, 127909; (c) Rollas, S.; Güniz Küçükgüzel, Ş. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939; (d) Etxebeste-Mitxeltorena, M.; Plano, D.; Astrain-Redin, N. New amides and phosphoramidates containing selenium: studies on their cytotoxicity and antioxidant activities in breast cancer. Antioxidants 2021, 10, 590; (e) Singh, U. S.; Mulamoottil, V. A.; Chu, C. K. 2’-Fluoro-6’-methylene carbocyclic adenosine and its phosphoramidate prodrug: A novel anti-HBV agent, active against drug-resistant HBV mutants. Med. Res. Rev. 2018, 38, 977–1002; (f) Wang, J.; Ansari, M. F.; Lin, J. M. Design and synthesis of sulfanilamide aminophosphonates as novel antibacterial agents towards Escherichia coli. Chin. J. Chem. 2021, 39, 2251–2263.
- 4(a) Hassen, Z.; Akacha, A. B.; Hajjem, B. Action des hydrazines fluoroalkylées sur les phosphoallènes: synthèse d’hydrazones N-fluoroalkylées β-phosphonatées. J. Fluorine Chem. 2003, 121, 177–183;
(b) Palacios, F.; Aparicio, D.; López, Y. Synthesis of functionalized α-amino-phosphine oxides and-phosphonates by addition of amines and aminoesters to 4-phosphinyl-and 4-phosphonyl-1,2-diaza-1,3- butadienes. Tetrahedron 2005, 61, 2815–2830;
(c) Uryupin, A. B.; Bodrin, G. V.; Goryunova, I. B. N’-Sulfonyl- and N’-Acylhydrazones of α- and β-Diphenylphosphorylalkanones: Synthesis and Structure. Russ. J. Gen. Chem. 2021, 91, 650–656.;
(d) ibiryakova, A. E.; Reznikov, A. N.; Rybakov, V. B. Enantioselective addition of β-keto phosphinate to ω-nitrostyrene in the presence of optically active nickel(II) complex. Russ. J. Org. Chem. 2017, 53, 153–156.
10.1134/S1070428017020014 Google Scholar
- 5 Zhou, S. F.; Li, D. P.; Liu, K.; Zou, J. P.; Asekun, O. T. Direct radical acetoxyphosphorylation of styrenes mediated by manganese(III). J. Org. Chem. 2015, 80, 1214–1220.
- 6 Xu, C. H.; Xiong, Z. Q.; Li, Y. Copper-catalyzed oxidative phosphonoheteroarylation of alkenes with phosphonates and N-heteroarenes via P–H/C–H functionalization. Org. Chem. Front. 2022, 9, 476–480.
- 7 Xu, J.; Li, X.; Gao, Y. Mn(III)-mediated phosphonation–azidation of alkenes: a facile synthesis of β-azidophosphonates. Chem. Commun. 2015, 51, 11240–11243.
- 8 Tao, Z. K.; Li, C. K.; Zhang, P. Z. Phosphinoyl radical-initiated 1,2-bifunctional thiocyanodiphenylphosphinoylation of alkenes. J. Org. Chem. 2018, 83, 2418–2424.
- 9 Shen, J.; Xiao, B.; Hou, Y. Cobalt(II)-Catalyzed Bisfunctionalization of Alkenes with Diarylphosphine Oxide and Peroxide. Adv. Synth. Catal. 2019, 361, 5198–5209.
- 10(a) Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Mechanistic studies in photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747; (b) Melchiorre, P. Introduction: photochemical catalytic processes. Chem. Rev. 2022, 122, 1483–1484; (c) Quintavalla, A.; Carboni, D.; Lombardo, M. Green Metrics and Sustainability in Photocatalysis. ChemCatChem 2024, 16, e202301225; (d) Chi, Y.; Wang, W.; Zhang, Q. Evaluation of practical application potential of a photocatalyst: ultimate apparent photocatalytic activity. Chemosphere 2021, 285, 131323.
- 11 Fu, Q.; Bo, Z. Y.; Ye, J. H.; Yu, D. g. Transition metal-free phosphonocarboxylation of alkenes with carbon dioxide via visible-light photoredox catalysis. Nat. Commun. 2019, 10, 3592.
- 12 Yang, H.; Li, M. M.; Zhang A; Ding, W. Visible-light-induced photocatalyst-and metal-free radical phosphinoyloximation of alkenes with tert-butyl nitrite as bifunctional reagent. Chin. Chem. Lett. 2025, 36, 110425.
- 13 Li, J. A.; Zhang, P. Z.; Liu, K.; Zou, J. P. Phosphinoyl radical-initiated α,β-aminophosphinoylation of alkenes. Org. Lett. 2017, 19, 4704–4706.
- 14(a) Gao, M. J.; Fan, J. H.; Liu, Y. Photocatalytic Radical Cascade Cyclization of N-(o-Cyanobiaryl) acrylamides with Sulfonyl Chlorides. J. Org. Chem. 2025, 90, 5008–5018; (b) Liu, X.; Yin, Y. L.; Jiang, Z. Y. Photoredox-catalysed formal [3 + 2] cycloaddition of N-aryl α-amino acids with isoquinoline N-oxides. Chem. Commun. 2019, 55, 11527–11530; (c) Liu, X. Q.; Chen, H.; Fan, J. H.; Liu, Y. Radical Cascade Cyclization of N-(o-Cyanobiaryl) acrylamides with Sulfonium Salts via Synergetic Photoredox and Copper Catalysis. Org. Lett. 2024, 26, 7650–7655; (d) Swierk, J. R. The cost of quantum yield. Org. Process Res. Dev. 2023, 27, 1411–1419.
- 15(a) Liu, X. Q.; Fan, J. H.; Tang, K. W.; Zhong, L. J.; Liu, Y. Visible-Light- Induced Copper-Catalyzed Alkylation/exo-Cyclization of 1,7-Dienes with Sulfonium Salts to Access Sulfur-Containing Polycyclic Compounds. Adv. Synth. Catal., 2024, 366, 3868–3874;
(b) Li, Q.; Zhao, X.; Yin, Y.; Jiang, Z. Asymmetric Photoredox Catalytic Minisci-Type Reactions of α-Bromide Amides. Org. Lett. 2025, 24, 1244–1249;
10.1021/acs.orglett.4c04791 Google Scholar(c) Lv, X.; Xu, H.; Yin, Y.; Jiang, Z. Visible Light-Driven Cooperative DPZ and Chiral Hydrogen-Bonding Catalysis. Chin. J. Chem. 2020, 38, 1480–1488.
- 16(a) Rathelot, P.; Azas, N.; El-Kashef, H. 1,3-Diphenylpyrazoles: synthesis and antiparasitic activities of azomethine derivatives. Eur. J. Med. Chem. 2002, 37, 671–679; (b) Ogundipe, O. O.; Shoberu, A.; Zou, J. P. Copper-catalyzed stereoselective radical phosphono-hydrazonation of alkynes. J. Org. Chem. 2022, 87, 14555–14564; (c) Huang, J.; Ouyang, L.; Li, J. B2pin2-mediated palladium-catalyzed diacetoxylation of aryl alkenes with O2 as oxygen source and sole oxidant. Org. Lett. 2018, 20, 5090–5093; (d) Majek, M.; Filace, F.; von Wangelin, A. J. On the mechanism of photocatalytic reactions with eosin Y. Beilstein J. Org. Chem. 2014, 10, 981–989; (e) Chen, X.; Chen, X.; Li, X. Acetonitrile-dependent oxyphosphorylation: A mild one-pot synthesis of β-ketophosphonates from alkenyl acids or alkenes. Tetrahedron 2017, 73, 2439–2446; (f) Nan, G. Yue, H. Direct synthesis of β-ketophosphine oxides via copper-catalyzed difunctionalization of alkenes with H-phosphine oxides and dioxygen. Tetrahedron Lett. 2018, 59, 2071–2074; (g) Zheng, L.; Cai, L.; Zhuo, X. Direct intermolecular three-component aminotrifluoromethylation of styrenes by visible-light-photoredox catalysis. Org. Chem. Front. 2022, 9, 6498–6504; (h) Yang, S.; Tan, X.; Liu, D. Visible Light-Induced Arylation/Alkylation/Phosphorylation of Isocyanides via EDA Complex Activation. Chin. J. Chem. 2025, 43, 1379–1384.




