Ketodeoxynonulosonic Acid Hydroxylase (Kdnase) Assisted Site-Specific Enzymatic α2,6-Sialylation
Yu Zhou
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
These authors contribute equally.
Search for more papers by this authorYun Li
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
These authors contribute equally.
Search for more papers by this authorJiayu Wen
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
Department of Pharmacy and Health Management, Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei, 050026 China
These authors contribute equally.
Search for more papers by this authorYan Zhang
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Zhifei Hu
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kan Zhong
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hongzhi Cao
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiansong Cheng
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorYu Zhou
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
These authors contribute equally.
Search for more papers by this authorYun Li
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
These authors contribute equally.
Search for more papers by this authorJiayu Wen
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
Department of Pharmacy and Health Management, Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei, 050026 China
These authors contribute equally.
Search for more papers by this authorYan Zhang
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Zhifei Hu
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kan Zhong
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hongzhi Cao
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotherapeutics, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiansong Cheng
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, 300353 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
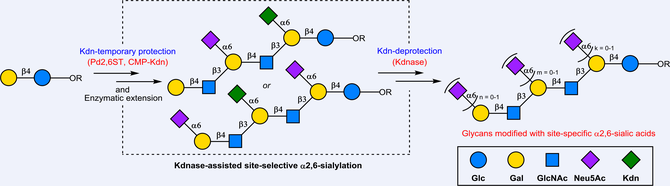
Owing to its promiscuous substrate specificity and high catalytic efficiency, the bacterial α2,6-sialyltransferase from Photobacterium damselae (Pd2,6ST) has been widely used for the synthesis of various α2,6-linked sialosides. However, Pd2,6ST is not a suitable enzyme for the regioselective α2,6-sialylation of complex acceptor substrates containing multiple galactose (Gal) and/or N-acetylgalactosamine (GalNAc) residues due to its promiscuous substrate specificity. In this study, a novel enzymatic substrate engineering strategy was developed to overcome this limitation by employing enzymatically introduced α2,6-linked ketodeoxynonulosonic acid (Kdn) as temporary “protecting group” at the unwanted sialylation sites. The Kdn “protecting group” can be selectively removed by a ketodeoxynonulosonic acid hydrolase from Aspergillus fumigatus (AfKdnase) at appropriate stage without affecting coexisting sialic acid residues, such as N-acetylneuraminic acid (Neu5Ac) or N-glycolylneuraminic acid (Neu5Gc). This strategy provides a general and practical approach for the synthesis of complex sialosides, including sialylated poly-LacNAc glycans, disialylated ganglioside glycan epitopes, and branched human milk oligosaccharides.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500146-sup-0001-supinfo.pdfPDF document, 17.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Varki, A. Sialic scids in human health and disease. Trends Mol. Med. 2008, 14, 351–360.
- 2
Ghosh, S. Sialic acid and biology of life: an introduction. In: Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease, Elsevier, London, 2020, pp. 1–61.
10.1016/B978-0-12-816126-5.00001-9 Google Scholar
- 3 Hu, Z.-F.; Zhong, K.; Cao, H. Recent rdvances in enzymatic and chemoenzymatic synthesis of N- and O-glycans. Curr. Opin. Chem. Biol. 2024, 78, 102417.
- 4 Wang, S.; Chen, C.; Gadi, M. R.; Saikam, V.; Liu, D.; Zhu, H.; Bollag, R.; Liu, K.; Chen, X.; Wang, F.; Wang, P. G.; Ling, P.; Guan, W.; Li, L. Chemoenzymatic modular assembly of O-GalNAc glycans for functional glycomics. Nat. Commun. 2021, 12, 3573.
- 5 Groux-Degroote, S.; Guérardel. Y.; Delannoy, P. Gangliosides: structures, biosynthesis, analysis, and roles in cancer. ChemBioChem 2017, 18, 1146–1154.
- 6 Hotta, K.; Komba, S.; Ishida, H.; Kiso, M.; Hasegawa, A. Synthetic studies on sialoglycoconjugates 61: synthesis of α-series ganglioside GM1α. J. Carbohydr. Chem. 1994, 13, 665–677.
- 7 Merrill, A. H. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422.
- 8 Ma, S.; Zhang, P.; Ye, J.; Tian, Y.; Tian, X.; Jung, J.; Macauley, M. S.; Zhang, J.; Wu, P.; Wen, L. Enzyme-sialylation-controlled chemical sulfation of glycan epitopes for decoding the binding of siglec ligands. J. Am. Chem. Soc. 2024, 146, 29469–29480.
- 9 Ma, Y.; Liu, Y.; Cao, C.; Peng, J.; Jiang, Y.; Li, T. Host–guest chemistry- mediated biomimetic chemoenzymatic synthesis of complex glycosphingolipids. J. Am. Chem. Soc. 2025, 147, 6974–6982.
- 10 Chen, X. Human milk oligosaccharides (HMOS): structure, function, and enzyme-catalyzed synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 113–190.
- 11 Bai, Y.; Agrahari, A. K.; Zhang, L.; Yu, H.; Yang, X.; Zheng, Z.; Su, W.; Fu, J.; Chen, X. EASyMap-guided stepwise one-pot multienzyme (StOPMe) synthesis and multiplex assays identify functional tetraose-core- human milk oligosaccharides. JACS Au 2025, 5, 822–837.
- 12 Bao, S.; Shen, T.; Shabahang, M. H.; Bai, G.; Li, L. Enzymatic synthesis of disialyllacto-N-tetraose (DSLNT) and related human milk oligosaccharides reveals broad siglec recognition of the atypical Neu5Acα2–6GlcNAc motif. Angew. Chem. Int. Ed. 2024, 63, e202411863.
- 13 Tseng, H.-K.; Lee, T.-Y.; Chiang, Y.-C.; Kuo W.-H; Tseng, H.-W.; Wang, H.-K.; Ni, C.-K.; Lin, C.-C. Versatile strategy for the chemoenzymatic synthesis of branched human milk oligosaccharides containing the lacto-N-biose motif. Angew. Chem. Int. Ed. 2024; 63, e202419021.
- 14 Tseng, H.-W.; Tseng, H.-K.; Ooi, K.-E.; You, C.-E.; Wang, H.-K.; Kuo, W.-H.; Ni, C.-K.; Manabe, Y.; Lin, C.-C. Controllable enzymatic synthesis of natural asymmetric human milk oligosaccharides. JACS Au 2024, 4, 4496–4506.
- 15 Schnaar, R. L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518.
- 16 Rodrigues, E.; Macauley, M. S. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers 2018, 10, 207.
- 17 Varki, A. Glycan-based interactions involving vertebrate sialic-acid- recognizing proteins. Nature 2007, 446, 1023–1029.
- 18 Ilver, D.; Johansson, P.; Miller-Podraza, H.; Nyholm, P. G.; Teneberg, S.; Karlsson, K. A. Bacterium–host protein–carbohydrate interactions. Methods Enzymol. 2003, 363, 134–157.
- 19 Rogers, G. N.; Paulson, J. C.; Daniels, R. S.; Skehel, J. J.; Wilson, I. A.; Wiley, D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983, 304, 76–78.
- 20 Komura, N.; Kato, K.; Udagawa, T.; Asano, S.; Tanaka, H. N.; Imamura, A.; Ishida, H.; Kiso, M.; Ando, H. Constrained sialic acid donors enable selective synthesis of α-glycosides. Science 2019, 364, 677–680.
- 21 Blixt, O.; Allin, K.; Pereira, L.; Datta, A.; Paulson, J. C. Efficient chemoenzymatic synthesis of O-linked sialyl oligosaccharides. J. Am. Chem. Soc. 2002, 124, 5739–5746.
- 22 Na, L.; Li, R.; Chen, X. Recent progress in synthesis of carbohydrates with sugar nucleotide-dependent glycosyltransferases. Curr. Opin. Chem. Biol. 2021, 61, 81–95.
- 23 Shivatare, S. S.; Chang, S.-H.; Tsai, T.-I.; Tseng, S. Y.; Shivatare, V. S.; Lin, Y.-S.; Cheng, Y.-Y.; Ren, C.-T.; Lee, C.-C. D.; Pawar, S.; Tsai, C.-S.; Shih, H.-W.; Zeng, Y.-F.; Liang, C.-H.; Kwong, P. D.; Burton, D. R.; Wu, C.-Y.; Wong, C.-H. Modular synthesis of N-glycans and arrays for the hetero-ligand binding analysis of HIV antibodies. Nat. Chem. 2016, 8, 338–346.
- 24 Wang, Z.; Chinoy, Z. S.; Ambre, S. G.; Peng, W.; McBride, R.; de Vries, R. P.; Glushka, J.; Paulson, J. C.; Boons, G.-J. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 2013, 341, 379–383.
- 25 Sugiarto, G.; Lau, K.; Qu, J.; Li, Y.; Lim, S.; Mu, S.; Ames, J. B.; Fisher, A. J.; Chen, X. A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating Lewis x. ACS Chem. Biol. 2012, 7, 1232–1240.
- 26 Yamamoto, T.; Nakashizuka, M.; Terada, I. Cloning and expression of a marine bacterial β-galactoside α2,6-sialyltransferase gene from Photobacterium damsela JT0160. J. Biochem. 1998, 123, 94–100.
- 27 Yu, C.-C. Withers, S. G. Recent developments in enzymatic synthesis of modified sialic acid derivatives. Adv. Synth. Catal. 2015, 357, 1633–1654.
- 28 Ma, W.; Xu, Z.; Jiang, Y.; Liu, J.; Xu, D.; Huang, W.; Li, T. Divergent enzymatic assembly of a comprehensive 64-membered IgG N-glycan library for functional glycomics. Adv. Sci. 2023, 10, 2303832.
- 29 Nycholat, C. M.; Peng, W.; McBride, R.; Antonopoulos, A.; de Vries, R. P.; Polonskaya, Z.; Finn, M. G.; Dell, A.; Haslam, S. M.; Paulson, J. C. Synthesis of biologically active N- and O-linked glycans with multisialylated poly-N-acetyllactosamine extensions using P. damsela α2–6-sialyltransferase. J. Am. Chem. Soc. 2013, 135, 18280–18283.
- 30 Prudden, A. R.; Liu, L.; Capicciotti, C. J.; Wolfert, M. A.; Wang, S.; Gao, Z.; Meng, L.; Moremen, K. W.; Boons, G.-J. Synthesis of Asymmetrical Multiantennary Human Milk Oligosaccharides. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 6954–6959.
- 31 Hidari, K. I.; Horie, N.; Murata, T.; Miyamoto, D.; Suzuki, T.; Usui, T.; Suzuki, Y. Purification and characterization of a soluble recombinant human ST6Gal I functionally expressed in Escherichia coli. Glycoconj. J. 2005, 22, 1–11.
- 32 Li, W.; McArthur, J. B.; Chen, X. Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res. 2019, 472, 86–97.
- 33 Yu, H.; Huang, S.; Chokhawala, H.; Sun, M.; Zheng, H.; Chen, X. Highly efficient chemoenzymatic synthesis of naturally cccurring and non- natural α2,6-linked sialosides: a P. damsela α2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. 2006, 45, 3938–3944.
- 34 Yu, H.; Lau, K.; Thon, V.; Autran, C. A.; Jantscher-Krenn E.; Xue, M.; Li, Y.; Go, S.; Qu, J.; Mu, S.; Ding, L.; Bode, L.; Chen, X. Synthetic disialyl hexasaccharides protect neonatal rats from necrotizing enterocolitis. Angew. Chem. Int. Ed. 2014, 53, 6687–6691.
- 35 Yu, H.; Yan, X.; Autran, C. A.; Li, Y.; Etzold, S.; Latasiewicz, J.; Robertson, B. M.; Li, J.; Bode, L.; Chen, X. Enzymatic and chemoenzymatic syntheses of disialyl glycans and their necrotizing enterocolitis preventing effects. J. Org. Chem. 2017, 82, 13152–13160.
- 36 Meng, X.; Yao, W.; Cheng, J.; Zhang, X.; Jin, L.; Yu, H.; Chen, X.; Wang, F.; Cao, H. Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J. Am. Chem. Soc. 2014, 136, 5205–5208.
- 37 Xu, Y.; Fan, Y.; Ye, J.; Wang, F.; Nie, Q.; Wang, L.; Wang, P. G.; Cao, H.; Cheng, J. Successfully engineering a bacterial sialyltransferase for regioselective α2,6-sialylation. ACS Catal. 2018, 8, 7222–7227.
- 38 Lu, N.; Ye, J.; Cheng, J.; Sasmal, A.; Liu, C.-C.; Yao, W.; Yan, J.; Khan, N.; Yi, W.; Varki, A.; Cao, H. Redox-controlled site-specific α2–6-sialylation. J. Am. Chem. Soc. 2019, 141, 4547–4552.
- 39 Nejatie, A.; Steves, E.; Gauthier, N.; Baker, J.; Nesbitt, J.; McMahon, S., A.; Oehler, V.; Thornton, N. J.; Noyovitz, B.; Khazaei, K.; Byers, B. W.; Zandberg, W. F.; Gloster, T. M.; Moore, M. M.; Benet, A. J. Kinetic and structural characterization of sialidases (Kdnases) from ascomycete fungal pathogens. ACS Chem. Biol. 2021, 16, 2632–2640.
- 40 Telford, J. C.; Yeung, J. H. F.; Xu, G.; Kiefel, M. J.; Watts, A. G.; Hader, S.; Chan, J.; Bebet, A. J.; Moore, M. M.; Taylor, G. L. The Aspergillus fumigatus sialidase is a 3-deoxy-D-glycero-D-galacto-2-nonulosonic acid hydrolase (KDNase). J. Biol. Chem. 2011, 286, 10783–10792.
- 41 Yeung, J. H. F.; Telford, J. C.; Shidmoossavee, F. S.; Bennet, A. J.; Taylor, G. L.; Moore, M. M. Kinetic and structural evaluation of selected active site mutants of the Aspergillus fumigatus KDNase (sialidase). Biochemistry 2013, 52, 9177–9186.
- 42 Fuchizawa, S.; Furuhata, K.; Matsuda, T.; Kitajima, K. Induction of KDNase Sm, a deaminoneuraminic acid (KDN) residue-specific sialidase from Sphingobacterium multivorum, using synthetic KDN-glycosides. Biochem. Biophys. Res. Commun. 1998, 248, 505–510.
- 43 Kitajima, K.; Kuroyanagi, H.; Inoue, S.; Ye, J.; Troy, F. A.; Inoue, Y. Discovery of a new type of sialidase, “KDNase,” which specifically hydrolyzes deaminoneuraminyl (3-deoxy-D-glycero-D-galacto-2- nonulosonic acid) but not N-acylneuraminyl linkages. J. Biol. Chem. 1994, 269, 21415–21419.
- 44 Li, Q.; Guo, Z. Enzymatic synthesis of glycosphingolipids: a review. Synthesis 2021, 53, 2367–2380.
- 45 Yan, J.; Li, J.; Hu, Z.-F.; Ma, X.; Li, Y.; Hu, X.; Ye, J.; Wang, W.; Wan, R.; Cao, H. Enzymatic synthesis of sialyl lactosamine grafted vhitooligosaccharides. Chin. J. Chem. 2023, 41, 1299–1304.
- 46 Fang, W.; Zhong, K.; Cheng, J.; Liu, X.-W.; Liu, C.-C.; Wang, Z.; Cao, H. Capture-release strategy facilitates rapid enzymatic assembly of oligosaccharides. Chin. J. Chem. 2021, 40, 343–350.
- 47 Li, Y.; Li, Y.; Guo, Y.; Chen, C.; Yang, L.; Jiang, Q.; Long, P.; Wang, S.; Li, L.; Fang, J. Enzymatic modular synthesis of asymmetrically branched human milk oligosaccharides. Carbohydr. Polym. 2024, 333, 121908.
- 48 Xia, H.; Zhong, K.; Li, Y.; Ye, J.; Wang, D.; Cai, C.; Mu.; W.; Liu, C.-C.; Cao, H. ACS Catal. 2024, 14, 13390–13399.
- 49 Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP–sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP–sialic acid synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435.
- 50 Zhai, Y.; Liang, M.; Fang, J.; Wang, X.; Guan, W.; Liu, X.-W.; Wang, P.; Wang, F. NahK/GlmU fusion enzyme: characterization and one-step enzymatic synthesis of UDP-N-acetylglucosamine. Biotechnol. Lett. 2012, 34, 1321–1326.
- 51 Peng, W.; Pranskevich, J.; Nycholat, C.; Gilbert, M.; Wakarchuk, W.; Paulson, J. C.; Razi, N. Helicobacter pylori β1,3-N-acetylglucosaminyltransferase for versatile synthesis of type 1 and type 2 poly-LacNAcs on N-linked, O-linked and I-antigen glycans. Glycobiology 2012, 22, 1453–1464.
- 52 Chen, X.; Fang, J.; Zhang, J.; Liu, Z.; Shao, J.; Kowal, P.; Andreana, P.; Wang, P. G. Sugar nucleotide regeneration beads (superbeads): a versatile tool for the practical synthesis of oligosaccharides. J. Am. Chem. Soc. 2001, 123, 2081–2082.
- 53 Muthana, M. M.; Qu, J.; Li, Y.; Zhang, L.; Yu, H.; Ding, L.; Malekan, H.; Chen, X. Efficient one-pot multienzyme synthesis of UDP-sugars using a promiscuous UDP-sugar pyrophosphorylase from Bifidobacterium longum (BLUSP). Chem. Commun. 2012, 48, 2728–2730.
- 54 Lau, K.; Thon, V.; Yu, H.; Ding, L.; Chen, Y.; Muthana, M. M.; Wong, D.; Huang, R.; Chen, X. Highly efficient chemoenzymatic synthesis of β1–4-linked galactosides with promiscuous bacterial β1–4-galactosyltransferases. Chem. Commun. 2010, 46, 6066–6068.
- 55 McArthur, J. B.; Yu, H.; Chen, X. A bacterial β1–3-galactosyltransferase enables multigram-scale synthesis of human milk lacto-N- tetraose (LNT) and its fucosides. ACS Catal. 2019, 9, 10721–10726.
- 56 Chen, X. Enabling chemoenzymatic strategies and enzymes for synthesizing sialyl glycans and sialyl glycoconjugates. Acc. Chem. Res. 2024, 57, 234–246.
- 57 Liu, C.-C.; Ye, J.; Cao, H. Chemical evolution of enzyme-catalyzed glycosylation. Acc. Chem. Res. 2024, 57, 636–647.




