Guest Molecule Induced FRET Enhancement of Photochromic Pillar[5]arene-Based Phenothiazine Derivatives
Corresponding Author
Hong Yao
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWenyu Cao
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorJinwang Wang
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorFeixiang Yang
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorShuning Qin
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorTai-Bao Wei
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorBingbing Shi
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorCorresponding Author
Qi Lin
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hong Yao
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWenyu Cao
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorJinwang Wang
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorFeixiang Yang
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorShuning Qin
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorTai-Bao Wei
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorBingbing Shi
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
Search for more papers by this authorCorresponding Author
Qi Lin
Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu, 730070 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
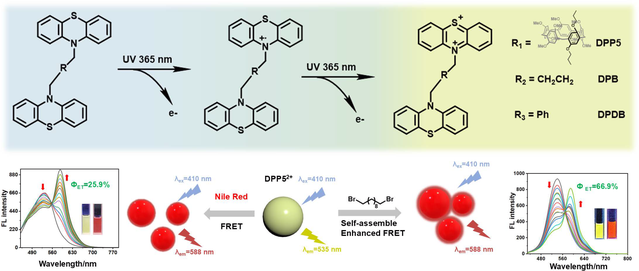
In recent years, the study of the photochromic behavior of phenothiazine derivatives has not only enriched the variety of color-changing materials but also provided new donor molecules for the construction of Förster Resonance Energy Transfer (FRET). This advancement broadens the application potential of photochromic materials and offers fresh perspective for FRET research. Herein, pillar[5]arene-linked biphenothiazine derivative (DPP5) was synthesized, while p-dibenzyl-linked biphenothiazine derivative (DPDB) and butyl-linked biphenothiazine derivative (DPB) were designed for comparative study. The photochromic behavior was demonstrated by UV-vis spectra, electron paramagnetic resonance (EPR) and chemical oxidation method, showing the transformation of DPP5 molecule into the radical cation DPP5•+ and subsequently into the dication DPP52+. Furthermore, a FRET system was constructed using dication species DPP52+ as the energy donor and Nile red (NiR) as the energy acceptor. The introduction of guest molecules, 1,6-dibromohexane (1,6-DBH) and 1,10-dibromodecane (1,10-DBD), into the above FRET system enhanced the energy transfer efficiency by increasing the aggregation degree of FRET system by utilizing the cavity of pillar[5]arene through host-guest interaction. The application of the photochromic behavior of phenothiazine derivatives into FRET system, along with the strategy of using guest molecule to enhance FRET properties, will contribute to the development of novel photochromic materials.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500040-sup-0001-supinfo.pdfPDF document, 3.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Tang, W.; Liang, P.; Wang, X.; Zhang, C.; Wang, G.; Liang, C.; Guan, M. Temporal Dynamic Photochromic Materials for Advanced Anticounterfeiting. J. Mater. Chem. C 2023, 11, 15169–15177.
- 2 Wang, J.; Jin, B.; Wang, N.; Peng, T.; Li, X.; Luo, Y.; Wang, S. Organoboron-Based Photochromic Copolymers for Erasable Writing and Patterning. Macromolecules 2017, 50, 4629–4638.
- 3 Song, Y.-P.; Zhang, J.-N.; Wang, J.-R.; Li, K.; Yuan, Y.-X.; Li, B.; Zang, S.-Q. A Fast Responsive Photochromic SCC-MOF for Photoswitching and Information Encryption. Sci. China Mater. 2024, 67, 698–704.
- 4 Li, X.; Liu, M.; Shao, J.; Sun, H.; Zhang, Q.; Peng, D.; Liu, F. High-Capacity Photochromic Rotary Encoder for Information Encryption and Storage. Adv. Funct. Mater. 2024, 34, 2402603.
- 5 Shi, Y.-S.; Yang, D.-D.; Xiao, T.; Fang, Y.-H.; Xia, Z.-G.; Zheng, X.-J. Naphthalenediimide-Based Photochromic MOFs: Structure Visualization upon Radical Formation, and Applications in Purple Light Detection, Inkless Printing and Anti-Counterfeiting. Chem. Eng. J. 2023, 462, 142275.
- 6 Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Induction of Skin Cancer by Long-Term Blue Light Irradiation. Biomedicines 2023, 11, 2321.
- 7 Exelby, R.; Grinter, R. Phototropy (or Photochromism). Chem. Rev. 1965, 65, 247–260.
- 8 Pardo, R.; Zayat, M.; Levy, D. Photochromic Organic–Inorganic Hybrid Materials. Chem. Soc. Rev. 2011, 40, 672–687.
- 9 Wu, R.-J.; Hsu, Y.-L.; Chou, W.-Y.; Cheng, H.-L. Enhancing Functionalities of Organic Ultraviolet-Visible Phototransistors Incorporating Spiropyran-Merocyanine Photochromic Materials. J. Mater. Chem. A 2021, 9, 22522–22532.
- 10 Zhang, R.; Jin, Y.; Yuan, L.; Deng, K.; Wang, C.; Xiong, G.; Chen, L.; Hu, Y. Inorganic Photochromism Material SrHfO3:Er3+ Integrating Multiple Optical Behaviors for Multimodal Anti-counterfeiting. J. Alloys Compd. 2022, 921, 166081.
- 11 Dong, X.; Guo, T.; Kitagawa, D.; Kobatake, S.; Palffy-Muhoray, P.; Bardeen, C. J. Performance of Composite Glass–Diarylethene Crystal Photomechanical Actuator Membranes. ACS Appl. Mater. Interfaces 2022, 14, 27149–27156.
- 12 Li, W.-B.; Chen, X.-H.; Chen, J.-Z.; Huang, R.; Ye, J.-W.; Chen, L.; Wang, H.-P.; Yang, T.; Tang, L.-Y.; Bai, J.; Mo, Z.-W.; Chen, X.-M. Photochromic Metal–Organic Framework for High-Resolution Inkless and Erasable Printing. ACS Appl. Mater. Interfaces 2022, 14, 8458–8463.
- 13 Sliman, H.; Dong, X.; Zhao, T. Functionalization of Polyethylene Terephthalate Knitted Fabric with Cowpea Protein and Biopolymer Complex: Applications for Enhancing Wettability and UV-Protection Properties. J. Colloid Interface Sci. 2020, 565, 360–367.
- 14 Revoju, S.; Matuhina, A.; Canil, L.; Salonen, H.; Hiltunen, A.; Abate, A.; Vivo, P. Structure-Induced Optoelectronic Properties of Phenothiazine-Based Materials. J. Mater. Chem. C 2020, 8, 15486–15506.
- 15 Gangadhar, P. S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine Functional Materials for Organic Optoelectronic Applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996.
- 16 Wang, N.; Guo, Z.; Ni, Z.; Xu, J.; Qiu, X.; Ma, J.; Wei, P.; Wang, Y. Molecular Tailoring of an n/p-type Phenothiazine Organic Scaffold for Zinc Batteries. Angew. Chem. Int. Ed. 2021, 60, 20826–20832.
- 17 Hou, J.-T.; Wang, B.; Zou, Y.; Fan, P.; Chang, X.; Cao, X.; Wang, S.; Yu, F. Molecular Fluorescent Probes for Imaging and Evaluation of Hypochlorite Fluctuations during Diagnosis and Therapy of Osteoarthritis in Cells and in a Mouse Model. ACS Sens. 2020, 5, 1949–1958.
- 18
Gao, M.; Tian, Y.; Li, X.; Gong, Y.; Fang, M.; Yang, J.; Li, Z. The Effect of Molecular Conformations and Simulated “Self-Doping” in Phenothiazine Derivatives on Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2023, 135, e202214908.
10.1002/ange.202214908 Google Scholar
- 19 Leitonas, K.; Tomkeviciene, A.; Baratte, G.; Dabuliene, A.; Punniyakoti, S. M.; Volyniuk, D.; Grazulevicius, J. V. Oxygen Sensing Properties of Thianthrene and Phenothiazine Derivatives Exhibiting Room Temperature Phosphorescence: Effect of Substitution of Phenothiazine Moieties. Sens. Actuators B Chem. 2021, 345, 130369.
- 20 Malinčík, J.; Kohout, M.; Svoboda, J.; Stulov, S.; Pociecha, D.; Böhmová, Z.; Novotná, V. Photochromic spiropyran-based liquid crystals. J. Mol. Liq. 2022, 346, 117842.
- 21 Sun, F.; Cai, J.; Wu, H.; Zhang, H.; Chen, Y.; Jiang, C.; Su, F.; Tian, Y.; Liu, Y. J. Novel Extended Viologen Derivatives for Photochromic and Electrochromic Dual-response Smart Windows. Sol. Energy Mater. Sol. Cells. 2023, 260, 112496.
- 22 Kitagawa, D.; Nakahama, T.; Nakai, Y.; Kobatake, S. 1,2-Diarylbenzene as Fast T-type Photochromic Switch. J. Mater. Chem. C 2019, 7, 2865–2870.
- 23 Zhang, W.; Li, W.; Song, Y.; Xu, Q.; Xu, H. Bacterial Detection based on Förster Resonance Energy Transfer. Biosens. Bioelectron. 2024, 255, 116244.
- 24 Fu, S.; Su, X.; Li, M.; Song, S.; Wang, L.; Wang, D.; Tang, B. Z. Controllable and Diversiform Topological Morphologies of Self-Assembling Supra-Amphiphiles with Aggregation-Induced Emission Characteristics for Mimicking Light-Harvesting Antenna. Adv. Sci. 2020, 7, 2001909.
- 25 Zhang, B.; Lyu, G.; Kelly, E. A.; Evans, R. C. Förster Resonance Energy Transfer in Luminescent Solar Concentrators. Adv. Sci. 2022, 9, 2201160.
- 26 Dey, S.; Kar, A. K. Enhanced Photoluminescence through Forster Resonance Energy Transfer in Polypyrrole-PMMA Blends for Application in Optoelectronic Devices. Mater. Sci. Semicond. Process. 2019, 103, 104644.
- 27 Lim, J.; Hwang, K. Y.; Kwak, S.-Y.; Cho, S. M.; Kim, J.-M.; Lee, J. Y. Enhanced Förster Energy Transfer Through Horizontal Orientation of Sensitizer Molecules in Hyperfluorescent Organic Light-Emitting Diodes. Adv. Opt. Mater. 2023, 11, 2300672.
- 28 dos Remedios, C. G.; Moens, P. D. J. Fluorescence Resonance Energy Transfer Spectroscopy Is a Reliable "Ruler" for Measuring Structural Changes in Proteins: Dispelling the Problem of the Unknown Orientation Factor. J. Struct. Biol. 1995, 115, 175–185.
- 29 Song, N.; Lou, X.-Y.; Yu, H.; Weiss, P. S.; Tang, B. Z.; Yang, Y.-W. Pillar[5]arene-based Tunable Luminescent Materials via Supramolecular Assembly-Induced Förster Resonance Energy Transfer Enhancement. Mater. Chem. Front. 2020, 4, 950–956.
- 30 Li, H.; Yang, J.; Li, D.; Li, X.; Li, J.; He, C. Host-Guest Approach to Promoting Photocatalysis Based on Consecutive Photo-Induced Electron-Transfer Processes via Efficient Förster Resonance Energy Transfer. Angew. Chem. Int. Ed. 2024, 63, e202409094.
- 31 Li, D.; Liu, X.; Yang, L.; Li, H.; Guo, G.; Li, X.; He, C. Highly Efficient Förster Resonance Energy Transfer between An Emissive Tetraphenylethylene-Based Metal–Organic Cage and the Encapsulated Dye Guest. Chem. Sci. 2023, 14, 2237–2244.
- 32 Qian, C.; Ma, Z.; Liu, J.; Zhang, X.; Wang, S.; Ma, Z. A Tri-state Fluorescent Switch with “Gated” Solid-state Photochromism Induced by an External Force. Chem. Asian J. 2021, 16, 3713–3718.
- 33
Ansaloni, L.; Deng, L. Advances in Polymer-Inorganic Hybrids as Membrane Materials. Recent Dev. Polym. Macro, Micro Nano Blends 2017, 7, 163.
10.1016/B978-0-08-100408-1.00007-8 Google Scholar
- 34 Khan, F.; Urbonas, E.; Volyniuk, D.; Grazulevicius, J. V.; Mobin, S. M.; Misra, R. White Hyperelectrofluorescence from Solution-Processable OLEDs Based on Phenothiazine Substituted Tetraphenylethylene Derivatives. J. Mater. Chem. C 2020, 8, 13375–13388.




