Selective Synthesis of Bromo-substituted 2H-Pyrroles and 3H-Pyrroles via Three-Component Cascade Annulation of 1,3-Enynes, NBS and TMSN3
Zhen Zhang
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorQingting Song
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorFan Qin
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorHan Yue
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorCorresponding Author
Xiaoyu Xie
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLei Wang
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Advanced Research Institute and Department of Chemistry, Taizhou University, Taizhou, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Tao Miao
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhen Zhang
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorQingting Song
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorFan Qin
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorHan Yue
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorCorresponding Author
Xiaoyu Xie
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLei Wang
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
Advanced Research Institute and Department of Chemistry, Taizhou University, Taizhou, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Tao Miao
Key Laboratory of Green and Precise Synthetic Chemistry and Application, Ministry of Education; Anhui Key Laboratory of Synthetic Chemistry and Applications, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
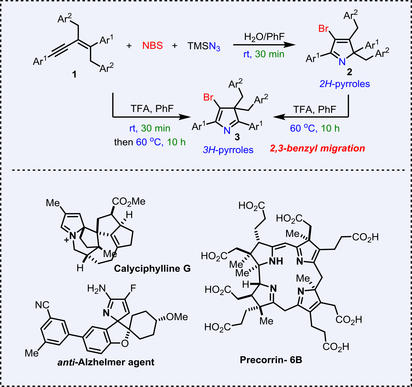
A novel and efficient method for selective synthesis of bromo-substituted 2H-pyrroles and 3H-pyrroles has been achieved from 1,3-enynes, NBS and TMSN3 via H2O-promoted cyclization reactions or TFA-catalyzed cyclization/2,3-shift reactions, providing a range of structurally diverse products in moderate to good yields under mild conditions.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401260-sup-0001-supinfo.pdfPDF document, 8.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected examples, see: (a) Shen, X.; Perry, T. L.; Dunbar, C. D.; Kelly-Borges, M.; Hamann, M. T. Debromosceptrin, an Alkaloid from the Caribbean Sponge Agelas Conifera. J. Nat. Prod. 1998, 61, 1302–1303; (b) Assmann, M.; Zea, S.; Kock, M. Sventrin, a New Bromopyrrole Alkaloid from the Caribbean Sponge Agelas Sventres. J. Nat. Prod. 2001, 64, 1593–1595; (c) Furstner, A. Chemistry and Biology of Roseophilin and the Prodigiosin Alkaloids: A Survey of the Last 2500 Years. Angew. Chem. Int. Ed. 2003, 42, 3582–3603; (d) Furstner, A.; Radkowski, K.; Peters, H. Chasing a Phantom by Total Synthesis: The Butylcycloheptylprodigios in Case. Angew. Chem. Int. Ed. 2005, 44, 2777–2781; (e) Vicente, J.; Vera, B.; Rodriguez, A. D.; Rodriguez-Escudero, I.; Raptis, R. G. Euryjanicin A: A New Cycloheptapeptide from the Caribbean Marine Sponge Prosuberites Laughlini. Tetrahedron Lett. 2009, 50, 4571–4574; (f) Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R. W.; de Voogd, N. J.; Wray, V.; Hentschel, U.; Kozytska, S.; Muller, W. E.; Proksch, P. From Anti-Fouling to Biofilm Inhibition: New Cytotoxic Secondary Metabolites from Two Indonesian Agelas Sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311; (g) Tilvi, S.; Moriou, C.; Martin, M. T.; Gallard, J. F.; Sorres, J.; Patel, K.; Petek, S.; Debitus, C.; Ermolenko, L.; Al-Mourabit, A. Agelastatin E, Agelastatin F, and Benzosceptrin C from the Marine Sponge Agelas Dendromorpha. J. Nat. Prod. 2010, 73, 720–723; (h) Sauleau, P.; Moriou, C.; Al Mourabit, A. Metabolomics Approach to Chemical Diversity of the Mediterranean Marine Sponge Agelas Oroides. Nat. Prod. Res. 2017, 31, 1625–1632.
- 2 Saito, S.; Kubota, T.; Kobayashi, J. i. Calyciphylline G, a Novel Alkaloid with an Unprecedented Fused-Hexacyclic Skeleton from Daphniphyllum Calycinum. Tetrahedron Lett. 2007, 48, 5693–5695.
- 3 Li, X.-L.; Wang, J.-Q.; Li, L.; Yin, Y.-W.; Ye, L.-W. Facile Synthesis of 2H-Pyrroles: Combination of Gold Catalysis and Lewis Acid Catalysis. Acta Chim. Sinica 2016, 74, 49–53.
- 4 Deery, E.; Schroeder, S.; Lawrence, A. D.; Taylor, S. L.; Seyedarabi, A.; Waterman, J.; Wilson, K. S.; Brown, D.; Geeves, M. A.; Howard, M. J.; Pickersgill, R. W.; Warren, M. J. An Enzyme-trap Approach Allows Isolation of Intermediates in Cobalamin Biosynthesis. Nat. Chem. Biol. 2012, 8, 933–940.
- 5(a) Polak, P.; Tobrman, T. Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles. Org. Lett. 2017, 19, 4608–4611; (b) Yamaguchi, M.; Fujiwara, S.; Manabe, K. Synthesis of 2,2,5-Trisubstituted 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles by Ligand-Controlled Site-Selective Dearomative C2-Arylation and Direct C3-Arylation. Org. Lett. 2019, 21, 6972–6977; (c) Zhuo, C.-X.; Zhou, Y.; You, S.-L. Highly Regio- and Enantioselective Synthesis of Polysubstituted 2H-Pyrroles via Pd-Catalyzed Intermolecular Asymmetric Allylic Dearomatization of Pyrroles. J. Am. Chem. Soc. 2014, 136, 6590–6593.
- 6 Bidusenko, I.; Schmidt, E.; Ushakov, I.; Vashchenko, A.; Trofimov, B. Base-Catalyzed [3+2] Cycloaddition of N-Benzyl Ketimines to Arylacetylenes Followed by Oxidation: A One-Pot Access to Polyarylated 2H-Pyrroles via Intermediate Pyrrolines. Org. Lett. 2021, 23, 4121–4126.
- 7 Liao, J.-Y.; Shao, P.-L.; Zhao, Y. Catalytic Divergent Synthesis of 3H or 1H Pyrroles by [3+2] Cyclization of Allenoates with Activated Isocyanides. J. Am. Chem. Soc. 2015, 137, 628–631.
- 8 Li, D.; Wang, L.; Yang, Y.; Zhang, M.; Peng, T.; Yang, D.; Wang, R. Construction of Optically Active 2H- and 3H-Pyrroles by Cyclization and Chirality Maintaining 1,5-Ester Shift Reactions. Adv. Synth. Catal. 2019, 361, 3744–3750.
- 9For selected examples, see: (a) Xiao, Y.; Zhang, J. Furans Versus 4H-Pyrans: Catalyst-Controlled Regiodivergent Tandem Michael Addition-Cyclization Reaction of 2-(1-Alkynyl)-2-Alken-1-Ones with 1,3-Dicarbonyl Compounds. Chem. Commun. 2009, 3594–3596; (b) Hu, J.; Liu, L.; Yang, S.; Liang, Y.-M. Phase-Transfer-Catalyzed Cyclization Reaction of Nucleophilic Addition to Electron-Deficient 1,3-Conjugated Enynes for the Synthesis of Functionalized 4H-Pyrans. Org. Biomol. Chem. 2011, 9, 3375–3379; (c) Zhou, W.; Yue, Z.; Zhang, J. A Highly Efficient One-Pot Trifluoromethylation/Cyclization Reaction of Electron-Deficient 1,3-Conjugated Enynes: Modular Access to Trifluoromethylated Furans and 2,3-Dihydrofurans. Org. Chem. Front. 2016, 3, 1416–1419; (d) Chang, Z. X.; Li, F. R.; Xia, C.; Li, F.; Li, H. S. Regioselective Access to 3-Ethylideneflavanones via Rhodium(I)-Catalyzed 1,3-Enyne Hydroacylation/Annulation Cascades. Adv. Synth. Catal. 2021, 363, 1722–1726; (e) He, Y.-C.; Yan, Y.-M.; Ren, Z.-X.; Wang, Y.-Z.; Yu, Q.; Xiong, J.; Wang, M.-L. Regioselective Synthesis of 2,3-Dihydrobenzo[f]-Isoindolones via Ag-Catalyzed Sequential Ugi 4cr/Cascade Radical Cyclization Reaction. Adv. Synth. Catal. 2021, 363, 1038–1043; (f) Ni, Q.; Wang, X.; Zeng, D.; Wu, Q.; Song, X. Organocatalytic Asymmetric Synthesis of Aza-Spirooxindoles via Michael/Friedel-Crafts Cascade Reaction of 1,3-Nitroenynes and 3-Pyrrolyloxindoles. Org. Lett. 2021, 23, 2273–2278; (g) Zhang, Z.; Li, A.; Zhao, B.; Li, P.; Wang, L.; Miao, T. Direct Synthesis of Sulfinylated Benzofulvenes via BF3·Et2O-Promoted Cascade Reactions of Arylsulfinic Acids with 1,3-Enynes. Org. Lett. 2021, 23, 8204–8208; (h) Zhao, B.; Zhang, Z.; Li, P.; Miao, T.; Wang, L. Synthesis of Spirolactones via a BF3·Et2O-Promoted Cascade Annulation of Alpha-Keto Acids and 1,3-Enynes. Org. Lett. 2021, 23, 5698–5702.
- 10For selected examples, see: (a) Bharathiraja, G.; Sakthivel, S.; Sengoden, M.; Punniyamurthy, T. A Novel Tandem Sequence to Pyrrole Syntheses by 5-endo-dig Cyclization of 1,3-Enynes with Amines. Org. Lett. 2013, 15, 4996–4999; (b) Guieu, B.; Le Roch, M.; David, M.; Gouault, N. Gold-Catalyzed Synthesis of Substituted 3-Trifluoromethylpyrroles from Mesylated Amino Trifluoromethyl-propargylic Alcohols. J. Org. Chem. 2017, 82, 13708–13713; (c) Huang, C.; Zeng, Y.; Cheng, H.; Hu, A.; Liu, L.; Xiao, Y.; Zhang, J. A One-Pot Construction of Halogenated Trifluoromethylated Pyrroles through NXS (X = Br, I) Triggered Cascade. Org. Lett. 2017, 19, 4968–4971; (d) He, H.; Lv, Y.; Hu, J.; Hou, Z.-W.; Wang, L. Seminormal-BrCH2CH2OH-Mediated Electrochemical Epoxidation of Unactivated Olefins. Green Chem. 2024, 26, 2157–2161.
- 11(a) Sun, C.-L.; Shi, Z.-J. Transition-Metal-Free Coupling Reactions. Chem. Rev. 2014, 114, 9219–9280;
(b) Wang, Z.; Gan, L.; Song, Z.; Liu, Y.; Wan, J.-P. tBuOK/TMSOK-Mediated “Alkyl Halide to Alkyl Free Radical” Transformation for Transition-Metal-Free Benzoin α-C-H Alkylation. Chin. J. Chem. 2024, 42, 3041–3046;
(c) Chaubey, T.; Borpatra, P.; Sharma, A.; Pandey, S.; Metal-Free Syntheses of α-Ketothioamide and α-Ketoamide Derivatives from Sulfoxonium Ylides. Org. Lett. 2022, 24, 8062–8066;
(d) Zhou, T.; Zhou, J.; Liu, Y.; Wan, J.-P.; Chen, F.-E. Transition Metal-free Tunable Synthesis of 3-(Trifluoromethylthio) and 3-Trifluoromethyl sulfinyl Chromones via Domino C-H Functionalization and Chromone Annulation of Enaminones. Chin. Chem. Lett. 2024, 35, 109683;
(e) Tian, L.; Wan, J.-P.; Liu, Y. Transition Metal-free Thiophene Construction in Pure Water by Multiplied C-H Functionalization with Enaminones and Elemental Sulfur. Green Synth. Catal. 2024, DOI: https://doi.org/10.1016/j.gresc.2024.09.002.
10.1016/j.gresc.2024.09.002 Google Scholar
- 12(a) Ross, S. D.; Finkelstein, M.; Petersen, R. C. Solvent Effects in the Reactions of N-Bromosuccinimide with Toluene, Fluorene and Acenaphthene; Evidence for a Polar Mechanism in Propylene Carbonate. J. Am. Chem. Soc. 1958, 80, 4327–4330; (b) Zhang, X.; Cao, W.-B.; Xu, X.-P.; Ji, S.-J. Treatment of Olefinic Amides with NBS in Water: Synthesis of Monobromo- and Multibromo-benzoxazines, Synthesis 2019, 51, 3805–3814; (c) Ding, R.; Li, Y.; Tao, C.; Cheng, B.; Zhai, H. Stereoselective Synthesis of (2Z)-2,4-Dienamides via NBS- Mediated Allyloxyl Addition−Claisen Rearrangement−Dehydrobromination Cascade Reaction of Ynsulfonamides. Org. Lett. 2015, 17, 3994–3997; (d) Huo, Z.; Gridnev, I.; Yamamoto, Y. A Method for the Synthesis of Substituted Quinolines via Electrophilic Cyclization of 1-Azido-2-(2-propynyl)benzene. J. Org. Chem. 2010, 75, 1266–1270; (e) Lu, Y.-H.; Wu, C.; Hou, J.-C.; Wu, Z.-L.; Zhou, M.-H.; Huang, X.-J.; He, W.-M. Ferrocene-Mediated Photocatalytic Annulation of N-Sulfonyl Ketimines. ACS Catal. 2023, 13, 13071–13076; (f) Tang, C.; Yuan, Y.; Jiao, N. Metal-Free Nitrogenation of 2-Acetylbiphenyls: Expeditious Synthesis of Phenanthridines. Org. Lett. 2015, 17, 2206–2209.




