Stereoselective Synthesis of 2-Deoxy Glycosides via a Novel 2-O-Resided (o-Alkynyl)benzoate-Initiated 1,2-Sulfur Migration/Glycosylation and Desulfurization Protocol as well as Mechanism Elucidation
Corresponding Author
Jin-Xi Liao
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]Search for more papers by this authorZhen-Qiang Li
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
Search for more papers by this authorYanli Qiu
Shanghai Frontiers Science Center of TCM Chemical Biology, Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorXiang-Yang Gao
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
Search for more papers by this authorXin Lv
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorHui Liu
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorYuan-Hong Tu
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorCorresponding Author
Jian-Song Sun
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
School of Life Science and Health Engineering, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jin-Xi Liao
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]Search for more papers by this authorZhen-Qiang Li
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
Search for more papers by this authorYanli Qiu
Shanghai Frontiers Science Center of TCM Chemical Biology, Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorXiang-Yang Gao
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
These authors contributed equally to this work.
Search for more papers by this authorXin Lv
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorHui Liu
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorYuan-Hong Tu
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
Search for more papers by this authorCorresponding Author
Jian-Song Sun
National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang, Jiangxi, 330022 China
School of Life Science and Health Engineering, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
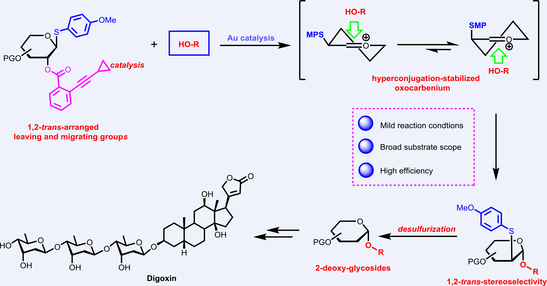
Stereoselective construction of 2-deoxy-glycosidic linkages has been achieved by the 1,2-sulfur migration/glycosylation and desulfurization strategy; however, current protocols suffer from harsh reaction conditions and unsatisfactory stereoselectivity, particularly during the 1,2-S-migration/glycosylation step. With 2-O-resided (o-alkynyl)benzoate and anomeric p-methoxyphenylsulfenyl groups as the initiating and migrating groups, respectively, a novel protocol for the efficient synthesis of 2-deoxy-glycosides via the 1,2-sulfur migration/glycosylation-desulfurization strategy has been established, which is featured by the mild and catalytic reaction conditions, expanded substrate scope, as well as good to excellent diastereoselectivity. Mechanism studies determined hyperconjugation-stabilized oxocarbenium ion as the key intermediate, achieving high 1,2-trans stereocontrol through thermodynamic, steric, as well as electrostatic effects. This provides the fresh insight for the operative mechanism of the 1,2-sulfur migration/glycosylation and desulfurization strategy, further corroborated by the elaborately designed testing reactions and DFT calculations. Moreover, the synthetic potential of the newly established protocol was examined by the practical synthesis of natural product, culminating in the acquisition of digoxin from acetylated digoxigenin in 25% overall yield through an 8-step longest linear sequence.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401198-sup-0001-supinfo.pdfPDF document, 11 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Elshahawi, S. I.; Shaaban, K. A.; Kharel, M. K.; Thorson, J. S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015, 44, 7591–7697.
- 2 Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137.
- 3 Bennett, C. S.; Galan, M. C. Methods for 2-deoxyglycoside synthesis. Chem. Rev. 2018, 118, 7931–7985.
- 4 Ryan, K. J.; Acton, E. M.; Goodman, L. Synthesis of 2-thio-D-ribose and 2’-thioadenosine derivatives. J. Org. Chem. 1971, 36, 2646–2657.
- 5 Auzanneau, F.-I.; Bundle, D. R. Incidence and avoidance of stereospecific 1,2-ethylthio group migration during the synthesis of ethyl 1-thio-α-L-rhamnopyranoside 2,3-orthoester. Carbohydr. Res. 1991, 212, 13–24.
- 6 Yu, B.; Yang, Z. A novel and expeditious approach to the stereoselective synthesis of 2-S-ethyl(phenyl)-2-deoxy-β-glycosides, ready precursors to 2-deoxy-β-glycosides. Tetrahedron Lett. 2000, 41, 2961–2964.
- 7 Zuurmond, H. M.; van der Klein, P. A. M.; van der Marel, G. A.; van Boom, J. H. Stereospecific 1,2-ethyl(phenyl)thio group migration in sugars: stereocontrolled synthesis of precursors to α- and β-2-deoxyglycosides. Tetrahedron Lett. 1992, 33, 2063–2066.
- 8 Zuurmond, H. M.; van der Klein, P. A. M.; van der Marel, G. A.; van Boom, J. H. A stereospecific approach towards the synthesis of 2-deoxy α- and β-glycosides based on a 1,2-ethyl (phenyl) thio group migration. Tetrahedron 1993, 49, 6501–6514.
- 9 Hou, D.; Lowary, T. L. Stereoselective synthesis of 2-deoxy-furonosides from 2,3-anhydro-furanosyl thioglycosides. Org. Lett. 2007, 9, 4487–4490.
- 10 Hou, D.; Lowary, T. L. 2,3-Anhydrosugars in glycoside bond synthesis. Application to 2,6-dideoxypyranosides. J. Org. Chem. 2009, 74, 2278–2289.
- 11 Yu, B.; Yang, Z. Stereoselective synthesis of 2-S-phenyl-2-deoxy-β- glycosides using 2,3-O-thiocarbonyl-1-thioglycoside donors via 1,2-migration concurrent glycosylation. Org. Lett. 2001, 3, 377–379.
- 12 Yu, B.; Wang, P. Efficient synthesis of the hexasaccharide fragment of landomycin A: using phenyl 2,3-O-thiocarbonyl-1-thioglycosides as 2-dexoy-β-glycoside precursors. Org. Lett. 2002, 4, 1919–1922.
- 13 Nicolaou, K. C.; Randall, L. J. L.; Chucholowski, A. Stereospecific 1,2-migrations in carbohydrates. Stereocontrolled synthesis of α- and β-deoxyglycosides. J. Am. Chem. Soc. 1986, 108, 2466–2467.
- 14 Viso, A.; Poopeiko, N.; Castillon, S. Stereoselective synthesis of nucleosides from 1-thio and 1-seleno glycosides through consecutive 1,2-migration and glycosylation under Mitsunobu conditions. Tetrahedron Lett. 2000, 41, 407–411.
- 15 Hou, D.; Taha, H. A.; Lowary, T. L. 2,3-Anhydrosugars in glycoside bond synthesis: mechanism of 2-deoxy-2-thioaryl glycoside formation. J. Am. Chem. Soc. 2009, 131, 12937–12948.
- 16 Hu, Y.; Yu, K.; Shi, L.-L.; Liu, L.; Sui, J.-J.; Liu, D.-Y.; Xiong, B.; Sun, J.-S. o-(p-Methoxyphenylethynyl)phenyl glycosides: versatile new glycosylation donors for highly efficient construction of glycosidic linkages. J. Am. Chem. Soc. 2017, 139, 12736–12744.
- 17 Liu, H.; Liang, Z.-F.; Liu, H.-J.; Liao, J.-X.; Zhong, L.-J.; Tu, Y.-H.; Zhang, Q.-J.; Xiong, B.; Sun, J.-S. ortho-Methoxycarbonylethynylphenyl thioglycosides (MCEPTs): versatile glycosyl donors enabled by electron-withdrawing substituents and catalyzed by gold(I) or Cu(II) complex. J. Am. Chem. Soc. 2023, 145, 3682–3695.
- 18 Chen, J.; Hansen, T.; Zhang, Q.-J.; Liu, D.-Y.; Sun, Y.; Yan, H.; Codee, J. D. C.; Schmidt, R. R.; Sun, J.-S. 1-Picolinyl-5-azido thiosialosides: versatile donors for the stereoselective construction of sialyl linkages. Angew. Chem. Int. Ed. 2019, 58, 17000–17008.
- 19 Yu, B. Gold(I)-catalyzed glycosylation with glycosyl o-alkynylbenzotes as donors. Acc. Chem. Res. 2018, 51, 507–516.
- 20Hashimoto, S.-i.; Yangiya, Y.; Honda, T.; Ikegami, S. A stereocontrolled construction of 2-deoxy-β-glycosidic linkages via 1,2-trans-β- glycosydation of 2-deoxy-2-[(p-methoxyphenyl)thio]glycopyranosyl N,N,N’,N’-tetramethylphosphoroamidates. Chem. Lett. 1992, 21, 1511–1514.
10.1246/cl.1992.1511 Google Scholar
- 21 Xiao K.; Hu, Y.; Wan, Y.; Li, X.; Niu, Q.; Yan, H.; Wang, L.; Liao, J.; Liu, D.; Tu, Y.; Sun, J.; Codee, J. D. C.; Zhang, Q. Hydrogen bond activated glycosylation under mild conditions. Chem. Sci. 2022, 13, 1600–1607.
- 22 Liu, H.; Liao, J.-X.; Hu, Y.; Tu, Y.-H.; Sun, J.-S. A highly efficient approach to construct (epi)-podophyllotoxin-4-O-glycosidic linkages as well as its application in concise syntheses of etoposide and teniposide. Org. Lett. 2016, 18, 1294–1297.
- 23 Jacobsson, M.; Malmberg, J.; Ellervik, U. Aromatic O-glycosylation. Carbohydr. Res. 2006, 341, 1266−1281.
- 24See Supporting Information.
- 25 Beaver, M. G.; Billings, S. B.; Woerpel, K. A. Nucleophilic substitution reactions of 2-phenylthio-substituted carbohydrate acetals and related systems: episulfonium ions vs. oxocarbenium ions as reactive intermediates. Eur. J. Org. Chem. 2008, 771−781.
- 26 Jones, D. K.; Liotta, D. C. Episulfonium ions may not be the stereodeterminants in glycosylations of 2-thioalkyl pyranosides. Tetrahedron Lett. 1993, 34, 7209−7212.
- 27 Ayala, L.; Lucero, C. G.; Romero, J. A. C.; Tabacco, S. A.; Woerpel, K. A. Stereochemistry of nucleophilic substitution reactions depending upon substituent: evidence for electrostatic stabilization of pseudoaxial conformers of oxocarbenium ions by heteroatom substituents. J. Am. Chem. Soc. 2003, 125, 15521−15528.
- 28 Sun, A.; Li, Z.; Wang, Y.; Meng, S.; Zhang, X.; Meng, X.; Li, S.; Li, Z.; Li, Z. Stereocontrolled synthesis of α-3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) glycosides using C3-p-tolylthio-substituted Kdo donors: access to highly branched Kdo oligosaccharides. Angew. Chem. Int. Ed. 2024, 63, e202313985.
- 29 van den Bos, L.; Codee, J. D. C.; Litjens, R. E. J. N.; Dinkelaar, J.; Overkleeft, H. S.; van der Marel, G. Uronic acids in oligosaccharide synthesis. Eur. J. Org. Chem. 2007, 3963–3976.
- 30 van der Vorm, S.; van Hengst, J. M. A.; Bakker, M.; Overkleeft, H. S.; van der Marel, G.; Codee, J. D. C. Mapping the relationship between glycosyl acceptor reactivity and glycosylation stereoselectivity. Angew. Chem. Int. Ed. 2018, 57, 8240–8244.
- 31 Fu, J.; Wu, Z.; Zhang, L. Clinical applications of the naturally occurring or synthetic glycosylated low molecular weight drugs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 487–522.
- 32 Liu, H.; Zhou, S.-Y.; Wen, G.-E.; Liu, X.-X.; Liu, D.-Y.; Zhang, Q.-J.; Schmidt, R. R.; Sun, J.-S. The 2,2-dimethyl-2-(ortho-nitrophenyl)acetyl (DMNPA) group: a novel protecting group in carbohydrate chemistry. Org. Lett. 2019, 21, 8049–8052.
- 33 Liu, H.; Hansen, T.; Zhou, S.-Y.; Wen, G.-E.; Liu, X.-X.; Zhang, Q.-J.; Codee, J. D. C.; Schmidt, R. R.; Sun, J.-S. Dual-participation protecting group solves the anomeric stereocontrol problems in glycosylation reactions. Org. Lett. 2019, 21, 8713–8717.
- 34 Liu, H.; Zhou, S.-Y.; Liao, J.-X.; Tu, Y.-H.; Sun, J.-S. Highly efficient synthesis of digoxin. Synlett 2021, 32, 810–813.




