Total Synthesis of the Conjugation-Ready Hexasaccharides of Pseudomonas aeruginosa Serotype O17 O-Antigen via One-Pot Glycosylation
Guangzong Tian
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorJialong Bao
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorGuodong Chen
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorXiaopeng Zou
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorChunjun Qin
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorJing Hu
Wuxi School of Medicine & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorCorresponding Author
Jian Yin
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
School of Life Sciences and Health Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]Search for more papers by this authorGuangzong Tian
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorJialong Bao
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorGuodong Chen
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorXiaopeng Zou
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorChunjun Qin
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorJing Hu
Wuxi School of Medicine & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorCorresponding Author
Jian Yin
School of Biotechnology and Key Laboratory of Carbohydrate Chemistry and Biotechnology of Ministry of Education & Innovation Center for Vaccine Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
School of Life Sciences and Health Engineering, Jiangnan University, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
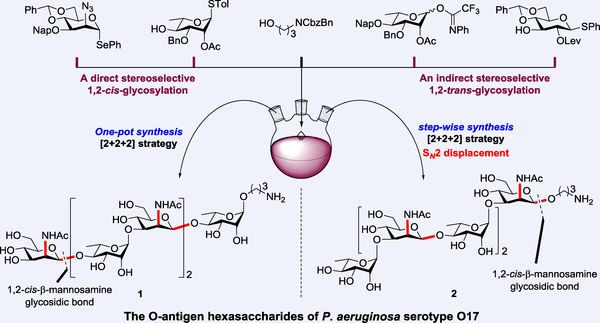
The eradication of Pseudomonas aeruginosa infections is becoming increasingly complex due to the emergence of multidrug-resistant strains, underscoring the urgent need for novel therapeutic strategies. Currently, no vaccine is available to prevent P. aeruginosa infections and the development of glycoconjugate vaccines based on P. aeruginosa lipopolysaccharides (LPS) presents significant challenges. To explore the immunological activity of the serotype O17 O-antigen, we present the first chemical synthesis of two hexasaccharides derived from the O17 O-antigen of P. aeruginosa, which possess distinct sequences. The synthesis of these two target hexasaccharides was accomplished using a chemoselective one-pot [2+2+2] assembly strategy and a common step-wise synthesis, respectively. The formation of β-mannosamine glycosidic linkages in products 1 and 2, was achieved through a direct stereoselective 1,2-cis-glycosylation involving 4,6-O-benzylidene-induced conformational locking facilitated by Ph2SO/Tf2O pre-activation, and an indirect 1,2-trans-β-glycosylation alongside SN2 substitution of azide groups at C2, respectively. The efficient synthesis of these conjugation-ready oligosaccharides from the O-antigen of P. aeruginosa serotype O17 will provide foundational materials for identifying key antigens and developing glycoconjugate vaccines.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401120-sup-0001-supinfo.pdfPDF document, 7.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Sainz-Mejías, M.; Jurado-Martín, I.; McClean, S. Understanding Pseudomonas aeruginosa-host Interactions: The ongoing Quest for an Efficacious Vaccine. Cells 2020, 9, 2617; (b) Azam, M. W.; Khan, A. U. Updates on the Pathogenicity Status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359.
- 2 Fujitani, S.; Sun, H. Y.; Yu, V. L.; Weingarten, J. A. Pneumonia due to Pseudomonas aeruginosa: Part I: Epidemiology, Clinical Diagnosis, and Source. Chest 2011, 139, 909–919.
- 3 Lambert, P. A. Mechanisms of Antibiotic Resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002, 95, 22–26.
- 4 Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. In WHO Bacterial Priority Pathogens List, World Health Organization, 2024.
- 5 Hoggarth, A.; Weaver, A.; Pu, Q.; Huang, T.; Schettler, J.; Chen, F.; Yuan, X.; Wu, M. Mechanistic Research Holds Promise for Bacterial Vaccines and Phage Therapies for Pseudomonas aeruginosa. Drug Des. Devel. Ther. 2019, 13, 909–924.
- 6 Pier, G. B. Pseudomonas aeruginosa Lipopolysaccharide: A Major Virulence Factor, Initiator of Inflammation and Target for Effective Immunity. Int. J. Med. Microbiol. 2007, 297, 277–295.
- 7(a) Liu, P. V.; Matsumoto, H.; Kusama, H.; Bergan, T. Survey of Heat-stable, Major Somatic Antigens of Pseudomonas aeruginosa. Int. J. Syst. Evol. Microbiol. 1983, 33, 256–264; (b) Liu, P.V.; Wang, S. Three New Major Somatic Antigens of Pseudomonas aeruginosa. J. Clin. Microbiol. 1990, 28, 922–925.
- 8 Pier, G. B.; Thomas, D.; Small, G.; Siadak, A.; Zweerink, H. In Vitro and In vivo Activity of Polyclonal and Monoclonal Human Immunoglobulins G, M, and A against Pseudomonas aeruginosa Lipopolysaccharide. Infect. Immun. 1989, 57, 174–179.
- 9(a) Sianturi, J.; Priegue, P.; Hu, J.; Yin, J.; Seeberger, P. H. Semi-synthetic Glycoconjugate Vaccine Lead Against Acinetobacter baumannii 17978. Angew. Chem. Int. Ed. 2022, 61, e202209556; (b) Rohokale, R.; Guo, Z. Development in the Concept of Bacterial Polysaccharide Repeating Unit-based Antibacterial Conjugate Vaccines. ACS Infect. Dis. 2023, 9, 178–212; (c) Tian, G.; Hu, J.; Qin, C.; Li, L.; Zou, X.; Cai, J.; Seeberger, P. H.; Yin, J. Chemical Synthesis and Immunological Evaluation of Helicobacter pylori Serotype O6 Tridecasaccharide O-Antigen Containing a DD-Heptoglycan. Angew. Chem. Int. Ed. 2020, 59, 13362–13370; (d) Zhu, Q.; Shen, Z.; Chiodo, F.; Nicolardi, S.; Yu, B. Chemical Synthesis of Glycans Up to a 128-mer Relevant to the O-Antigen of Bacteroides vulgatus. Nat. Commun. 2020, 11, 4142; (e) Yao, W.; Xiong, D. C.; Yang, Y.; Geng, C.; Cong, Z.; Li, F.; Li, B.; Qin, X.; Wang, L.; Xue, W.; Yu, N.; Zhang, H.; Wu, X.; Liu, M.; Ye, X. Automated Solution-phase Multiplicative Synthesis of Complex Glycans up to a 1,080-mer. Nat. Synth. 2022, 1, 854–863; (f) Tian, G.; Hu, J.; Qin, C.; Li, L.; Ning, Y.; Zhu, S.; Xie, S.; Zou, X.; Seeberger, P. H.; Yin, J. Chemical Synthesis and Antigenicity Evaluation of an Aminoglycoside Trisaccharide Repeating Unit of Pseudomonas aeruginosa Serotype O5 O-Antigen Containing a Rare Dimeric-ManpN3NA. J. Am. Chem. Soc. 2024, 146, 18427–18439; (g) Zhao, M.; Tian, G.; Qin, C.; Zou, X.; Seeberger, P. H.; Hu, J.; Yin, J. Immunological Exploration of Helicobacter pylori Serotype O2 O-Antigen by Using a Synthetic Glycan Library. Chin. J. Chem. 2024, 42, 243–251; (h) Hao, T.; Feng, K.; Jin, H.; Li, J.; Zhou, C.; Liu, X.; Zhao, W.; Yu, F.; Li, T. Acceptor-Reactivity-Controlled Stereoconvergent Synthesis and Immunological Activity of a Unique Pentasaccharide from the Cell Wall Polysaccharide of Cutibacterium acnes C7. Angew. Chem. Int. Ed. 2024, 63, e202405297; (i) Li, T.; Wang, J.; Zhu, X.; Zhou, X.; Sun, S.; Wang, P.; Cao, H.; Yu, G.; Li, M. Synthesis of Rare 6-Deoxy-D-/ L-Heptopyranosyl Fluorides: Assembly of a Hexasaccharide Corresponding to Campylobacter jejuni Strain CG8486 Capsular Polysaccharide. J. Am. Chem. Soc. 2021, 143, 11171–11179; (j) Liu, C.-C.; Huo, C.-X.; Zhai, C.; Zheng, X.-J.; Xiong, D.-C.; Ye, X.-S. Synthesis and Immunological Evaluation of Pentamannose-Based HIV-1 Vaccine Candidates. Bioconjugate Chem. 2022, 33, 807−820.
- 10 Wang, Z.; Liu, X.; Dacanay, A.; Harrison, B. A.; Fast, M.; Colquhoun, D. J.; Lund, V.; Brown, L. L.; Li, J.; Altman, E. Carbohydrate Analysis and Serological Classification of Typical and Atypical Isolates of Aeromonas salmonicida: A Rationale for the Lipopolysaccharide-based Classification of A. salmonicida. Fish Shellfish Immun. 2007, 23, 1095–1106.
- 11 Knirel, Y. A.; Kochetkov, N. K. The Structure of Lipopolysaccharides of Gram-negative Bacteria. III. The Structure of O-Antigens: A Review. Biochem.-Moscow. 1994, 59, 1325–1383.
- 12 Zähringer, U.; Rettenmaier, H.; Senchenkova, S. N.; Knirel, Y. A. Structure of a New 6-Deoxy-alpha-D-talan from Burkholderia (Pseudomonas) plantarii Strain DSM 6535, which is Different from the O-Chain of the Lipopolysaccharide. Carbohyd. Res. 1997, 300, 143–151.
- 13 Cox, A. D.; Wilkinson, S. G. Structures of the O-Specific Polymers from the Lipopolysaccharides of the Reference Strains for Pseudomonas cepacia Serogroups O3 and O5. Carbohyd. Res. 1989, 195, 123–129.
- 14 Knirel, Y. A.; Kocharova, N. A.; Shashkov, A. S.; Varbanets, L. D.; Kochetkov, N. K.; Stanislavsky, E. S.; Mashilova, G. M. Antigenic polysaccharides of bacteria. 17. Structure of O-Specific Polysaccharide Chain of Pseudomonas aeruginosa X (Meitert) Lipopolysaccharide. Bioorg. Khim. 1986, 12, 1268–1273.
- 15(a) Alex, C.; Demchenko, A. V. Recent Advances in Stereocontrolled Mannosylation: Focus on Glycans Comprising Acidic and/or Amino Sugars. Chem. Rec. 2021, 21, 3278–3294; (b) Sasaki, K.; Tohda, K. Recent Topics in β-Stereoselective Mannosylation. Tetrahedron Lett. 2018, 59, 496–503; (c) Liu, X.; Lin, Y.; Peng, W.; Zhang, Z.; Gao, L.; Zhou, Y.; Song, Z.; Wang, Y.; Xu, P.; Yu, B.; Sun, H.; Xie, W.; Li, W. Direct Synthesis of 2,6-Dideoxy-β-glycosides and β-Rhamnosides with a Stereodirecting 2-(Diphenylphosphinoyl)acetyl Group. Angew. Chem. Int. Ed. 2022, 61, e202206128.
- 16(a) Crich, D.; Sun, S. Direct Formation of β-Mannopyranosides and other Hindered Glycosides from Thioglycosides. J. Am. Chem. Soc. 1998, 120, 435–436; (b) Huang, M.; Garrett, G.; Birlirakis, E. N.; Bohé, L.; Pratt, D. A.; Crich, D. Dissecting the Mechanisms of a Class of Chemical Glycosylation Using Primary 13C Kinetic Isotope Effects. Nat. Chem. 2012, 4, 663–667; (c) Crich, D.; Sharma, D. Is Donor−acceptor Hydrogen Bonding Necessary for 4,6-O-Benzylidene-directed β-Mannopyranosylation? Stereoselective Synthesis of β-C-Mannopyranosides and α-C-Glucopyranosides. Org. Lett. 2008, 10, 4731–4734.
- 17(a) Yasomanee, J. P.; Demchenko, A. V. Effect of Remote Picolinyl and Picoloyl Substituents on the Stereoselectivity of Chemical Glycosylation. J. Am. Chem. Soc. 2012, 134, 20097–20102; (b) Pistorio, S. G.; Yasomanee, J. P.; Demchenko, A. V. Hydrogen-bond-mediated Aglycone Delivery: Focus on β-Mannosylation. Org. Lett. 2014, 16, 716–719
- 18(a) Rai, D.; Kulkarni, S. S. Total Synthesis of Trisaccharide Repeating Unit of Staphylococcus aureus Type 8 (CP8) Capsular Polysaccharide. Org. Lett. 2023, 25, 1509–1513; (b) Cai, J.; Hu, J.; Qin, C.; Li, L.; Shen, D. Tian, G.; Zou, X.; Seeberger, P. H.; Yin, J. Chemical Synthesis Elucidates the Key Antigenic Epitope of the Autism-related Bacterium Clostridium bolteae Capsular Octadecasaccharide. Angew. Chem. Int. Ed. 2020, 59, 20529–20537; (c) Zhang, S.; Seeberger, P. H. Total Syntheses of Conjugation-ready Repeating Units of Acinetobacter baumannii AB5075 for Glycoconjugate Vaccine Development. Chem.-Eur. J. 2021, 27, 17444–17451.
- 19 Kaji, E.; Anabuki, N.; Zen, S. Syntheses of Three Interglycosidic Isomers of N-Acetyl-β-D-mannosaminyl-L-rhamnoses Associated with O-Antigens of Several Gram-negative Opportunistic Pathogens. Chem. Pham. Bull. 1995, 43, 1441–1447.
- 20(a) Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P-H.; Hung, S-C. “One-Pot” Protection, Glycosylation, and Protection-Glycosylation Strategies of Carbohydrates. Chem. Rev. 2018, 118, 8025–8104; (b) Chen, Z.; Xiao, G. Total Synthesis of Nona-decasaccharide Motif from Ganoderma sinense Polysaccharide Enabled by Modular and One-Pot Stereoselective Glycosylation Strategy. J. Am. Chem. Soc. 2024, 146, 17446–17455. (c) Ma, Y.; Zhang, Y.; Huang, Y.; Chen, Z.; Xian, Q.; Su, R.; Jiang, Q.; Wang, X.; Xiao, G. One-Pot Assembly of Mannose-Capped Lipoarabinomannan Motifs up to 101-Mer from the Mycobacterium tuberculosis Cell Wall. J. Am. Chem. Soc. 2024, 146, 4112–4122.
- 21 Mironov, Y. V.; Sherman, A. A.; Nifantiev, N. E. Homogeneous Azidophenylselenylation of Glycals Using TMSN3-Ph2Se2-PhI(OAc)2. Tetrahedron Lett. 2004, 45, 9107–9110.
- 22(a) Yu, B.; Tao, H. Glycosyl Trifluoroacetimidates. Part 1: Preparation and Application as New Glycosyl Donors. Tetrahedron Lett. 2001, 42, 2405–2407; (b) Yu, B.; Sun, J.; Yang, X. Assembly of Naturally Occurring Glycosides, Evolved Tactics, and Glycosylation Methods. Acc. Chem. Res. 2012, 45, 1227–1236.
- 23 Pal, D.; Mukhopadhyay, B. Chemical Synthesis of β-L-Rhamnose Containing the Pentasaccharide Repeating Unit of the O-Specific Polysaccharide from a Halophilic Bacterium Halomonas ventosae RU5S2EL in the Form of its 2-Aminoethyl Glycoside. J. Org. Chem. 2021, 86, 8683–8694.
- 24 d’Ortoli, T. A.; Widmalm, G. Synthesis of the Tetrasaccharide Glycoside Moiety of Solaradixine and Rapid NMR-based Structure Verification Using the Program CASPER. Tetrahedron, 2016, 72, 912–927.
- 25 Hagen, B.; Ali, S.; Overkleeft, H. S.; van der Marel, G. A.; Codée, J. D. C. Mapping the Reactivity and Selectivity of 2-Azidofucosyl Donors for the Assembly of N-Acetylfucosamine-containing Bacterial Oligosaccharides. J. Org. Chem. 2017, 82, 848–868.
- 26(a) Yang, X.; Zhang, H.; Zhao, Q.; Li, Q.; Li, T.; Gao, J. Total Synthesis of the Repeating Units of Highly Functionalized O-Antigens of Pseudomonas aeruginosa ATCC 27577, O10, and O19. JACS Au 2024, 4, 2351−2362; (b) Sun, W.; Tian, G.; Ding, M.; Qin, C.; Zou, X.; Hu, J.; Yin, J. Chemical Synthesis of a Key Precursor Relevant to the Tetrasaccharide Repeating Unit from Treponema medium ATCC 700293. Chin. J. Chem. 2024, 42, 1615–1622; (c) Pradhan, K.; Paul, A.; Rai, D.; Mishra, A. K.; Balhara, P.; Kulkarni, S. S. Total Synthesis of Vibrio Cholerae O43 Tetrasaccharide Repeating Unit. J. Org. Chem. 2024, 89, 4019−4030.




