Chiral Porous Frameworks for Enantioselective Separation and Asymmetric Catalysis
Bing Yan
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorJin-Hui Song
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorDa-Long Zhang
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Zong-Jie Guan
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Innovation Institute of Industrial Design and Machine Intelligence Quanzhou-Hunan University, Quanzhou, Fujian, 362801 China
Research Institute of Hunan University in Chongqing, Chongqing, 401120 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu Fang
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]Search for more papers by this authorBing Yan
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorJin-Hui Song
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorDa-Long Zhang
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Zong-Jie Guan
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Innovation Institute of Industrial Design and Machine Intelligence Quanzhou-Hunan University, Quanzhou, Fujian, 362801 China
Research Institute of Hunan University in Chongqing, Chongqing, 401120 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu Fang
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
The preparation and resolution of chiral molecules hold significant importance in scientific and industrial domains, such as drug development and manufacturing. In recent years, chiral porous frameworks have attracted increasing attention in asymmetric catalysis and enantiomer resolution due to their excellent performance. The metal-organic frameworks (MOFs), covalent organic frameworks (COFs), porous organic cages (POCs), and porous coordination cages (PCCs) are important representative of the porous framework family. Significantly, chirality can be easily introduced into these framework materials through simple bottom-up or post-modification methods, thereby promoting their applications related to chirality. In this review, we systematically summarize the synthesis strategies of four classes of chiral framework materials and their applications in asymmetric catalysis and enantiomeric resolution. Finally, we present some perspectives on the future development in chiral porous frameworks.
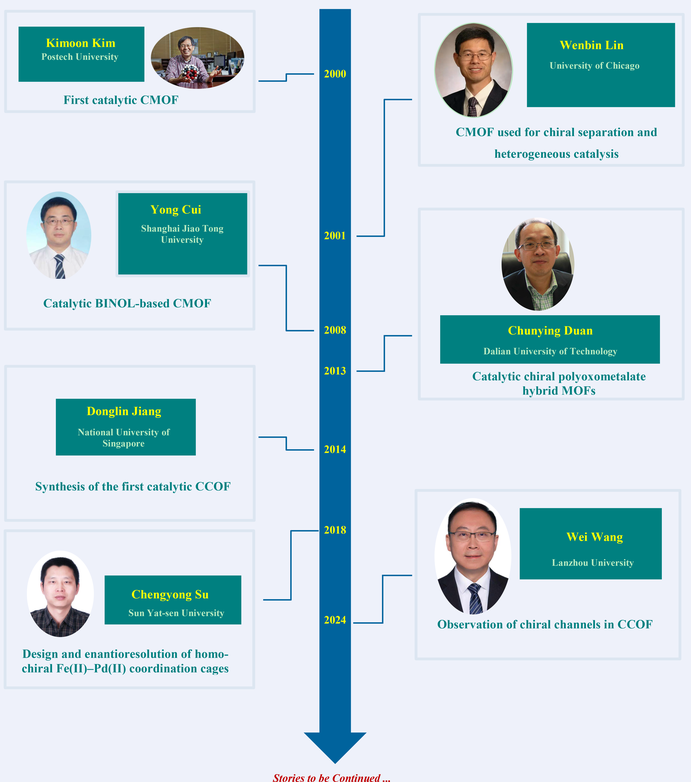
Key Scientists
Significant progress has been made in the development of chiral porous frameworks, primarily driven by the application of chiral molecules. This area of research has seen contributions from many distinguished scientists. A particularly important milestone was reached in 2000, when Kimoon Kim reported the first catalytic Chiral Metal-Organic Framework (CMOF). In 2001, Lin et al. reported a new generation of recyclable CMOF capable of chiral separation and heterogeneous catalysis. Simultaneously, researchers such as Cui and Duan have made substantial contributions, advancing the field considerably. Furthermore, notable developments have been made in the area of Chiral Covalent Organic Frameworks (CCOFs), with pioneering work by researchers like Jiang, Wang, and Cui. Meanwhile, some groups such as Su and Li have made significant strides in the chiral cages. These remarkable accomplishments have drawn considerable interest.
References
- 1 Gong, W.; Wang, W.; Dong, J.; Pan, X.; Liu, Y.; Yang, H.-B.; Fu, X.; Cui, Y. Recent Progress of Chiral Hierarchical Assemblies from a Chinese Perspective. CCS Chem. 2023, 5, 2736–2759.
- 2 Brewer, A.; Davis, A. P. Chiral Encoding May Provide a Simple Solution to the Origin of Life. Nat. Chem. 2014, 6, 569–574.
- 3 Shen, J.; Okamoto, Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2015, 116, 1094–1138.
- 4 Dong, J.; Liu, Y.; Cui, Y. Artificial Metal-Peptide Assemblies: Bioinspired Assembly of Peptides and Metals through Space and across Length Scales. J. Am. Chem. Soc. 2021, 143, 17316–17336.
- 5 Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144.
- 6 Han, X.; Yuan, C.; Hou, B.; Liu, L.; Li, H.; Liu, Y.; Cui, Y. Chiral Covalent Organic Frameworks: Design, Synthesis and Property. Chem. Soc. Rev. 2020, 49, 6248–6272.
- 7 Han, Z.; Shi, W.; Cheng, P. Synthetic Strategies for Chiral Metal-Organic Frameworks. Chin. Chem. Lett. 2018, 29, 819–822.
- 8 Hao, Y.; Lu, Y. L.; Jiao, Z.; Su, C. Y. Photocatalysis Meets Confinement: An Emerging Opportunity for Ohotoinduced Organic Transformations. Angew. Chem. Int. Ed. 2024, 63, e202317808.
- 9 Li, Y.; Li, S.; Huang, F.; Zhang, Z.; Jin, J. N.; Tan, M.; Chen, L. Synthesis and Application of Chiral Organic Imine Molecular Cages. Chin. J. Org. Chem. 2024, 44, 2617–2639.
- 10 Liu, B.-H.; Zong, Y.; Liu, N.; Wu, Z.-Q. Advances in Self-assembly-Based Circularly Polarized Luminescent Materials. Sci. China Chem. 2024, 67, 3247–3257.
- 11 Lu, Y.; Zhang, H.; Zhu, Y.; Marriott, P. J.; Wang, H. Emerging Homochiral Porous Materials for Enantiomer Separation. Adv. Funct. Mater. 2021, 31, 2101335.
- 12 Shen, Y.; Wu, W.; Yu, Z.; Yang, C. Recent Advances in Post-Chiroptical Manipulation of Supramolecular Aggregates Assembled with Molecular Modules. Sci. China Chem. 2024, 67, 2842–2863.
- 13 Sun, Z.; Hou, J.; Li, L.; Tang, Z. Nanoporous Materials for Chiral Resolution. Coord. Chem. Rev. 2020, 425, 213481.
- 14 Wang, J.-Q.; Han, X.-N.; Han, Y.; Chen, C.-F. Advances in Circularly Polarized Luminescence Materials Based on Chiral Macrocycles. Chem. Commun. 2023, 59, 13089–13106.
- 15 Zhang, H.; Lou, L. L.; Yu, K.; Liu, S. Advances in Chiral Metal-Organic and Covalent Organic Frameworks for Asymmetric catalysis. Small 2021, 2005, 686.
- 16 Zhao, J.; Zeng, K.; Jin, T.; Dou, W.-T.; Yang, H.-B.; Xu, L. Circularly Polarized Luminescence in Macrocycles and Cages: Ddesign, Preparation, and Application. Coord. Chem. Rev. 2024, 502, 215598.
- 17 Zhou, M.; El-Sayed, E.-S. M.; Ju, Z.; Wang, W.; Yuan, D. The Synthesis and Applications of Chiral Pyrrolidine Functionalized Metal-Organic Frameworks and Covalent-Organic Frameworks. Inorg. Chem. Front. 2020, 7, 1319–1333.
- 18 Zhang, L.; Liu, H.; Yuan, G.; Han, Y. F. Chiral Coordination Metallacycles/Metallacages for Enantioselective Recognition and Separation. Chin. J. Chem. 2021, 39, 2273–2286.
- 19 Liang, Y.; Li, E.; Wang, K.; Guan, Z.-J.; He, H.-H.; Zhang, L.; Zhou, H.-C.; Huang, F.; Fang, Y. Organo-Macrocycle-Containing Hierarchical Metal-Organic Frameworks and Cages: Design, Structures, and Applications. Chem. Soc. Rev. 2022, 51, 8378–8405.
- 20 Liang, Y.; Yang, X.; Wang, X.; Guan, Z.-J.; Xing, H.; Fang, Y. A Cage-on-MOF Strategy to Coordinatively Functionalize Mesoporous MOFs for Manipulating Selectivity in Adsorption and Catalysis. Nat. Commun. 2023, 14, 5223.
- 21
Peng, Y.; Guan, Z.-J.; Liu, K.-K.; Zhang, D.; Zhao, Y.; Jin, M.; Wang, S.; Fang, Y. Porous Covalent Cages for Visualizing the Adsorbed Iodine and Stabilizing Ylide Intermediates. CCS Chem. DOI: https://doi.org/10.31635/ccschem.024.202404689.
10.31635/ccschem.024.202404689 Google Scholar
- 22 Peng, Y.; Su, Z.; Jin, M.; Zhu, L.; Guan, Z.-J.; Fang, Y. Recent Advances in Porous Molecular Cages for Photocatalytic Organic Conversions. Dalton Trans. 2023, 52, 15216–15232.
- 23 Montà-González, G.; Sancenón, F.; Martínez-Máñez, R.; Martí-Centelles, V. Purely Covalent Molecular Cages and Containers for Guest Encapsulation. Chem. Rev. 2022, 122, 13636–13708.
- 24 Ham, R.; Nielsen, C. J.; Pullen, S.; Reek, J. N. H. Supramolecular Coordination Cages for Artificial Photosynthesis and Synthetic Photocatalysis. Chem. Rev. 2023, 123, 5225–5261.
- 25 Saha, R.; Mondal, B.; Mukherjee, P. S. Molecular Cavity for Catalysis and Formation of Metal Nanoparticles for Use in Catalysis. Chem. Rev. 2022, 122, 12244–12307.
- 26 Seo, J. S.; Whang, D.; Lee, H.; Jun, S. I.; Oh, J.; Jeon, Young. J.; Kim, K. A Homochiralmetal-Organic Porous Material for Enantioselective Separation and Catalysis. Nature 2000, 404, 982–986.
- 27 Cho, S.-H.; Ma, B.; Nguyen, S. T.; Hupp, J. T.; Albrecht-Schmitt, T. E. A Metal-Organic Framework Material that Functions as an Enantioselective Catalyst for Olefin Epoxidation. Chem. Commun. 2006, 2563–2565.
- 28 Song, F.; Wang, C.; Flkowski, J. M.; Ma, L.; Lin, W. Isoreticular Chiral Metal−Organic Frameworks for Asymmetric Alkene Epoxidation: Tuning Catalytic Activity by Controlling Framework Catenation and Varying Open Channel Sizes. J. Am. Chem. Soc. 2010, 132, 15390–15398.
- 29 Wu, K.; Li, K.; Hou, Y.-J.; Pan, M.; Zhang, L.-Y.; Chen, L.; Su, C.-Y. Homochiral D4-symmetric Metal–Organic Cages from Stereogenic Ru(II) Metalloligands for Effective Enantioseparation of Atropisomeric Molecules. Nat. Commun. 2016, 7, 10487.
- 30 Li, G.; Yu, W.; Cui, Y. A Homochiral Nanotubular Crystalline Framework of Metallomacrocycles for Enantioselective Recognition and Separation. J. Am. Chem. Soc. 2008, 130, 4582–4583.
- 31 Dong, J.; Tan, C.; Zhang, K.; Liu, Y.; Low, P. J.; Jiang, J.; Cui, Y. Chiral NH-controlled Supramolecular Metallacycles. J. Am. Chem. Soc. 2017, 139, 1554–1564.
- 32 Dong, J.; Liu, L.; Tan, C.; Xu, Q.; Zhang, J.; Qiao, Z.; Chu, D.; Liu, Y.; Zhang, Q.; Jiang, J.; Han, Y.; Davis, A. P.; Cui, Y. Free-Standing Homochiral 2D Monolayers by Exfoliation of Molecular Crystals. Nature 2022, 602, 606–611.
- 33 Wang, Z.; Cohen, S. M. Postsynthetic Covalent Modification of a Neutral Metal−Organic Framework. J. Am. Chem. Soc. 2007, 129, 12368–12369.
- 34 Xu, H.; Chen, X.; Gao, J.; Lin, J.; Addicoat, M.; Irle, S.; Jiang, D. Catalytic Covalent Organic Frameworks via Pore Surface Engineering. Chem. Commun. 2014, 50, 1292–1294.
- 35 Tan, C.; Han, X.; Li, Z.; Liu, Y.; Cui, Y. Controlled Exchange of Achiral Linkers with Chiral Linkers in Zr-Based UiO-68 Metal-Organic Framework. J. Am. Chem. Soc. 2018, 140, 16229–16236.
- 36 Zhang, S.-Y.; Li, D.; Guo, D.; Zhang, H.; Shi, W.; Cheng, P.; Wojtas, L.; Zaworotko, M. J. Synthesis of a Chiral Crystal Form of MOF-5, CMOF-5, by Chiral Induction. J. Am. Chem. Soc. 2015, 137, 15406–15409.
- 37 Wu, D.; Zhou, K.; Tian, J.; Liu, C.; Tian, J.; Jiang, F.; Yuan, D.; Zhang, J.; Chen, Q.; Hong, M. Induction of Chirality in a Metal-Organic Framework Built from Achiral Precursors. Angew. Chem. Int. Ed. 2020, 60, 3087–3094.
- 38 Han, X.; Zhang, J.; Huang, J.; Wu, X.; Yuan, D. Liu, Y.; Cui, Y. Chiral Induction in Covalent Organic Frameworks. Nat. Commun. 2018, 9, 1294.
- 39 Luo, D.; Wang, X.-Z.; Yang, C.; Zhou, X.-P.; Li, D. Self-Assembly of Chiral Metal-Organic Tetartoid. J. Am. Chem. Soc. 2017, 140, 118–121.
- 40 Evans, O. R. Ngo, H. L.; Lin, W. Chiral Porous Solids Based on Lamellar Lanthanide Phosphonates. J. Am. Chem. Soc. 2001, 123, 10395–10396.
- 41 Wu, C.-D.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal-Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941.
- 42 Sawano, T.; Thacker, N. C.; Lin, Z.; McIsaac, A. R.; Lin, W. Robust, Chiral, and Porous BINAP-Based Metal-Organic Frameworks for Highly Enantioselective Cyclization Reactions. J. Am. Chem. Soc. 2015, 137, 12241–12248.
- 43 Falkowski, J. M.; Sawano, T.; Zhang, T.; Tsun, G.; Chen, Y.; Lockard, J. V.; Lin, W. Privileged Phosphine-Based Metal-Organic Frameworks for Broad-Scope Asymmetric Catalysis. J. Am. Chem. Soc. 2014, 136, 5213–5216.
- 44 Ma, L.; Falkowski, J. M.; Abney, C.; Lin, W. A Series of Isoreticular Chiral Metal-Organic Frameworks as A Tunable Platform for Asymmetric Catalysis. Nat. Chem. 2010, 2, 838–846.
- 45 Dong, D.; Wu, P.; He, C.; Xie, Z.; Duan, C. Homochiral Metal-Organic Frameworks for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2010, 132, 14321–14323.
- 46 Jing, X.; He, C.; Dong, D.; Yang, L.; Duan, C. Homochiral Crystallization of Metal-Organic Silver Frameworks: Asymmetric [3+2] Cycloaddition of an Azomethine Ylide. Angew. Chem. Int. Ed. 2012, 51, 10127–10131.
- 47 Xu, H.; Gao, J.; Jiang, D. Stable, Crystalline, Porous, Covalent Organic Frameworks as a Platform for Chiral Organocatalysts. Nat. Chem. 2015, 7, 905–912.
- 48 Xu, H.-S.; Ding, S.-Y.; An, W.-K.; Wu, H.; Wang, W. Constructing Crystalline Covalent Organic Frameworks from Chiral Building Blocks. J. Am. Chem. Soc. 2016, 138, 11489–11492.
- 49 Wang, L. K.; Zhou, J. J.; Lan, Y. B.; Ding, S. Y.; Yu, W.; Wang, W. Divergent Synthesis of Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 9443–9447.
- 50 Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285.
- 51 Ma, H.-C.; Kan, J.-L.; Chen, G.-J.; Chen, C.-X.; Dong, Y.-B. Pd NPs-Loaded Homochiral Covalent Organic Framework for Heterogeneous Asymmetric Catalysis. Chem. Mater. 2017, 29. 6518–6524.
- 52 Ma, H.-C.; Zhao, C.-C.; Chen, G.-J.; Dong, Y.-B. Photothermal Conversion Triggered Thermal Asymmetric Catalysis within Metal Nanoparticles Loaded Homochiral Covalent Organic Framework. Nat. Commun. 2019, 10, 3368.
- 53 Kang, X.; Cheng, C.; Chen, X.; Dong, J.; Liu, Y.; Cui, Y. Three-Dimensional Homochiral Covalent Organic Frameworks with Intrinsic Chiral qzd Topology. J. Am. Chem. Soc. 2024, 146, 8407–8416.
- 54 Sun, J.-K.; Zhan, W.-W.; Akita, T.; Xu, Q. Toward Homogenization of Heterogeneous Metal Nanoparticle Catalysts with Enhanced Catalytic Performance: Soluble Porous Organic Cage as a Stabilizer and Homogenizer. J. Am. Chem. Soc. 2015, 137, 7063–7066.
- 55 Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating Highly Catalytically Active Metal Nanoclusters Inside Porous Organic Cages. Nat. Catal. 2018, 1, 214–220.
- 56 Liu, C.; Liu, K.; Wang, C.; Liu, H.; Wang, H.; Su, H.; Li, X.; Chen, B.; Jiang, J. Elucidating Heterogeneous Photocatalytic Superiority of Microporous Porphyrin Organic Cage. Nat. Commun. 2020, 11, 1047.
- 57 Mondal, B.; Acharyya, K.; Howlader, P.; Mukherjee, P. S. Molecular Cage Impregnated Palladium Nanoparticles: Efficient, Additive-Free heterogeneous Catalysts for Cyanation of Cryl Halides. J. Am. Chem. Soc. 2016, 138, 1709–1716.
- 58 Bierschenk, S. M.; Pan, J. Y.; Settineri, N. S.; Warzok, U.; Bergman, R. G.; Raymond, K. N.; Toste, F. D. Impact of Host Flexibility on Selectivity in a Supramolecular Host-Catalyzed Enantioselective aza-Darzens Reaction. J. Am. Chem. Soc. 2022, 144, 11425–11433.
- 59 Zhao, C.; Sun, Q.-F.; Hart-Cooper, W. M.; DiPasquale, A. G.; Toste, F. D.; Bergman, R. G.; Raymond, K. N. Chiral Amide Directed Assembly of a Diastereo and Enantiopure Supramolecular Host and its Application to Enantioselective Catalysis of Neutral Substrates. J. Am. Chem. Soc. 2013, 135, 18802–18805.
- 60 Brown, C. J.; Bergman, R. G.; Raymond, K. N. Enantioselective Catalysis of the aza-Cope Rearrangement by a Chiral Supramolecular Assembly. J. Am. Chem. Soc. 2009, 131, 17530–17531.
- 61 Peng, Y.; Gong, T.; Zhang, K.; Lin, X.; Liu, Y.; Jiang, J.; Cui, Y. Engineering Chiral Porous Metal-Organic Frameworks for Enantioselective Adsorption and Separation. Nat. Commun. 2014, 5, 4406.
- 62 Das, M. C.; Guo, Q.; He, Y.; Kim, J.; Zhao, C.-G.; Hong, K.; Xiang, S.; Zhang, Z.; Thomas, K. M.; Krishna, R.; Chen, B. Interplay of Metalloligand and Organic Ligand to Tune Micropores within Isostructural Mixed-Metal Organic Frameworks (M-MOFs) for Their Highly Selective Separation of Chiral and Achiral Small Molecules. J. Am. Chem. Soc. 2012, 134, 8703–8710.
- 63 Li, G.; Yu, W.; Ni, J.; Liu, T.; Liu, Y.; Sheng, E.; Cui, Y. Self-Assembly of a Homochiral Nanoscale Metallacycle from a Metallosalen Complex for Enantioselective Separation. Angew. Chem. Int. Ed. 2008, 47, 1245–1249.
- 64 Suh, K.; Yutkin, M. P.; Dybtsev, D. N.; Fedin, V. P.; Kim, K. Enantioselective Sorption of Alcohols in a Homochiral Metal-Organic Framework. Chem. Commun. 2012, 48, 513–515.
- 65 Bradshaw, D.; Prior, T. J.; Cussen, E. J.; Claridge, J. B.; Rosseinsky, M. J. Permanent Microporosity and Enantioselective Sorption in a Chiral Open Framework. J. Am. Chem. Soc. 2004, 126, 6106–6114.
- 66 Guo, J.; Zhang, Y.; Zhu, Y.; Long, C.; Zhao, M.; He, M.; Zhang, X.; Lv, J.; Han, B.; Tang, Z. Ultrathin Chiral Metal-Organic-Framework Nanosheets for Efficient Enantioselective Separation. Angew. Chem. Int. Ed. 2018, 57, 6873–6877.
- 67 Li, G.; Yu, W.; Ni, J.; Liu, T.; Liu, Y.; Sheng, E.; Cui, Y. Self-Assembly of a Homochiral Nanoscale Metallacycle from a Metallosalen Complex for Enantioselective Separation. Angew. Chem. Int. Ed. 2008, 47, 1245–1249.
- 68 Xie, S.-M.; Zhang, Z.-J.; Wang, Z.-Y.; Yuan, L.-M. Chiral Metal-Organic Frameworks for High-Resolution Gas Chromatographic Separations. J. Am. Chem. Soc. 2011, 133, 11892–11895.
- 69 Zhang, S.-Y.; Yang, C.-X.; Shi, W.; Yan, X.-P.; Cheng, P.; Wojtas, L.; Zaworotko, M. J. A Chiral Metal-Organic Material that Enables Enantiomeric Identification and Purification. Chem 2017, 3, 281–289.
- 70 Nuzhdin, A. L.; Dybtsev, D. N.; Bryliakov, K. P.; Talsi, E. P.; Fedin, V. P. Enantioselective Chromatographic Resolution and One-Pot Synthesis of Enantiomerically Pure Sulfoxides over a Homochiral Zn-organic Framework. J. Am. Chem. Soc. 2007, 129, 12958–12959.
- 71 Tanaka, K.; Muraoka, T.; Hirayama, D.; Ohnish, A. Highly Efficient Chromatographic Resolution of Sulfoxides Using a New Homochiral MOF-Silica Composite. Chem. Commun. 2012, 48, 8577–8579.
- 72 Wang W.; Dong, X.; Nan, J.; Jin, W.; Hu, Z.; Chen Y. A Homochiral Metal-Organic Framework Membrane for Enantio selective Separation. Chem. Commun. 2012, 48, 7022–7024.
- 73 Lu, Y.; Zhang, H.; Chan, J. Y.; Ou, R.; Zhu, H.; Forsyth, M.; Marijanovic, E. M.; Doherty, C. M.; Marriott, P. J.; Holl, M. M. B.; Wang, H. Homochiral Mof-Polymer Mixed Matrix Membranes for Efficient Separation of Chiral Molecule. Angew. Chem. Int. Ed. 2019, 58, 16928–16935.
- 74 Qian, H.-L.; Yang, C.-X.; Yan, X.-P. Bottom-Up Synthesis of Chiral Covalent Organic Frameworks and Their Bound Capillaries for Chiral Separation. Nat. Commun. 2016, 7, 12014.
- 75 Zhang, S.; Zheng, Y.; An, H.; Aguila, B.; Yang, C. X.; Dong, Y.; Xie, W.; Cheng, P.; Zhang, Z.; Chen, Y.; Ma, S. Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation. Angew. Chem. Int. Ed. 2018, 57, 16754–16759.
- 76 Yuan, C.; Jia, W.; Yu, Z.; Li, Y.; Zi, M.; Yuan, L.-M.; Cui, Y. Are Highly Stable Covalent Organic Frameworks the Key to Universal Chiral Stationary Phases for Liquid and Gas Chromatographic Separations? J. Am. Chem. Soc. 2022, 144, 891–900.
- 77 Zhang, J.-H.; Xie, S.-M.; Chen, L.; Wang, B.-J.; He, P.-G.; Yuan, L.-M. Homochiral Porous Organic Cage with High Selectivity for the Separation of Racemates in Gas Chromatography. Anal. Chem. 2015, 87, 7817–7824.
- 78 Kewley, A.; Stephenson, A.; Chen, L.; Briggs, M. E.; Hasell, T.; Cooper, A. I. Porous Organic Cages for Gas Chromatography Separations. Chem. Mater. 2015, 27, 3207–3210.
- 79 Chen, Y.; Wu, G.; Chen, B.; Qu, H.; Jiao, T.; Li, Y.; Ge, C.; Zhang, C.; Liang, L.; Zeng, X.; Cao, X.; Wang, Q.; Li, H. Self-Assembly of a Purely Covalent Cage with Homochirality by Imine Formation in Water. Angew. Chem. Int. Ed. 2021, 60, 18815–18820.
- 80 Tan, C.; Jiao, J.; Li, Z.; Liu, Y.; Han, X.; Cui, Y. Design and Assembly of a Chiral Metallosalen-Based Octahedral Coordination Cage for Supramolecular Asymmetric Catalysis. Angew. Chem. Int. Ed. 2018, 57, 2085–2090.
- 81 Howlader, P.; Zangrando, E.; Mukherjee, P. S. Self-Assembly of Enantiopure Pd12 Tetrahedral Homochiral Nanocages with Tetrazole Linkers and Chiral Recognition. J. Am. Chem. Soc. 2020, 142, 9070–9078.
- 82 Howlader, P.; Mondal, S.; Ahmed, S.; Mukherjee, P. S. Guest-Induced Enantioselective Self-Assembly of a Pd6 Homochiral Octahedral Cage with a C3-symmetric Pyridyl Donor. J. Am. Chem. Soc. 2020, 142, 20968–20972.
- 83 Zhu, C.; Tang, H.; Yang, K.; Fang, Y.; Wang, K.-Y.; Xiao, Z.; Wu, X.; Li, Y.; Powell, J. A.; Zhou, H.-C. Homochiral Dodecanuclear Lanthanide “Cage in Cage” for Enantioselective Separation. J. Am. Chem. Soc. 2021, 143, 12560–12566.




