Catalytic Asymmetric Conjugate Addition and Allylic Substitution of Cyclobutenones with Arylzinc Halides
Renming Pan
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Ping Lu
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorRenming Pan
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Ping Lu
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
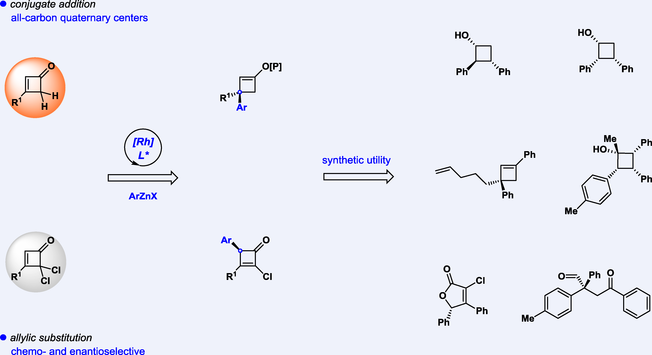
Conjugate addition and allylic substitution are two essential chemical transformations, and they could be competitive for substrates with multiple reactive sites. Herein, we report the diversified enantioselective synthesis of cyclobutenes via the functionalization of cyclobutenones. The conjugate addition of cyclobutenones with arylzinc halides provided enantioenriched cyclobutenes with all-carbon quaternary centers. On the other hand, when cyclobutenones with gem-dichloro groups were employed, a chemo- and enantioselective allylic substitution occurred. Further synthetic utility was demonstrated for synthesizing versatile cyclobutane derivatives, together with ring-opening and expansion products.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400882-sup-0001-supinfo.pdfPDF document, 14.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Harutyunyan, S.; Hartog, T.; Geurts, K.; Minnaard, A.; Feringa, B. Catalytic Asymmetric Conjugate Addition and Allylic Alkylation with Grignard Reagents. Chem. Rev. 2008, 108, 2824–2852; (b) Alexakis, A.; Backvall, J.; Krause, N.; Pamies, O.; Dieguez, M. Enantioselective Copper-Catalyzed Conjugate Addition and Allylic Substitution Reactions. Chem. Rev. 2008, 108, 2796–2823; (c) Howell, G. Asymmetric and Diastereoselective Conjugate Addition Reactions: C–C Bond Formation at Large Scale. Org. Process Res. Dev. 2012, 16, 1258–1272.
- 2(a) Hawner, C.; Alexakis, A. Metal-catalyzed Asymmetric Conjugate Addition Reaction: Formation of Quaternary Stereocenters. Chem. Commun. 2010, 46, 7295–7306; (b) Feng, J.; Holmes, M.; Krische, M. Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev. 2017, 117, 12564–12580.
- 3(a) Lee, K.; Brown, M.; Hird, A.; Hoveyda, A. A Practical Method for Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers through NHC-Cu-Catalyzed Conjugate Additions of Alkyl- and Arylzinc Reagents to β-Substituted Cyclic Enones. J. Am. Chem. Soc. 2006, 128, 7182–7184; (b) Brown, M.; May, T.; Baxter, C.; Hoveyda, A. All-Carbon Quaternary Stereogenic Centers by Enantioselective Cu-Catalyzed Conjugate Additions Promoted by a Chiral N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2007, 46, 1097–1100.
- 4(a) Hawner, C.; Li, K.; Cirriez, V.; Alexakis, A. Copper-Catalyzed Asymmetric Conjugate Addition of Aryl Aluminum Reagents to Trisubstituted Enones: Construction of Aryl-Substituted Quaternary Centers. Angew. Chem. Int. Ed. 2008, 47, 8211–8214; (b) May, T.; Brown, M.; Hoveyda, A. Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers by Catalytic Asymmetric Conjugate Additions of Alkyl and Aryl Aluminum Reagents to Five-, Six-, and Seven-Membered-Ring β-Substituted Cyclic Enones. Angew. Chem. Int. Ed. 2008, 47, 7358–7362.
- 5(a) Susse, L.; Stoltz, B. Enantioselective Formation of Quaternary Centers by Allylic Alkylation with First-Row Transition-Metal Catalysts. Chem. Rev. 2021, 121, 4084–4099;
(b) Fletcher, S.; Goetzke, F. Additions to Racemates: A Strategy for Developing Asymmetric Cross-Coupling Reactions. Synlett 2021, 32, 1816–1825.
10.1055/s-0040-1706033 Google Scholar
- 6 Perez, M.; Fananas-Mastral, M.; Bos, P.; Rudolph, A.; Harutyunyan, S.; Feringa, B. Catalytic Asymmetric Carbon–Carbon Bond Formation via Allylic Alkylations with Organolithium Compounds. Nat. Chem. 2011, 3, 377–381.
- 7(a) Alexakis, A.; Hajjaji, S.; Polet, D.; Rathgeb, X. Iridium-Catalyzed Asymmetric Allylic Substitution with Aryl Zinc Reagents. Org. Lett. 2007, 9, 3393–3395; (b) Polet, D.; Rathgeb, X.; Falciola, C.; Langlois, J.; Hajjaji, S.; Alexakis, A. Enantioselective Iridium-Catalyzed Allylic Arylation. Chem. Eur. J. 2009, 15, 1205–1216.
- 8 Selim, K.; Matsumoto, Y.; Yamada, K.; Tomioka, K. Efficient Chiral N-Heterocyclic Carbene/Copper(I)-Catalyzed Asymmetric Allylic Arylation with Aryl Grignard Reagents. Angew. Chem. Int. Ed. 2009, 48, 8733–8735.
- 9 Gao, F.; Lee, Y.; Mandai, K.; Hoveyda, A. Quaternary Carbon Stereogenic Centers through Copper-Catalyzed Enantioselective Allylic Substitutions with Readily Accessible Aryl- or Heteroaryllithium Reagents and Aluminum Chlorides. Angew. Chem. Int. Ed. 2010, 49, 8370–8374.
- 10(a) Guduguntla, S.; Hornillos, V.; Tessier, R.; Fananas-Mastral, M.; Feringa, B. Chiral Diarylmethanes via Copper-Catalyzed Asymmetric Allylic Arylation with Organolithium Compounds. Org. Lett. 2016, 18, 252–255; (b) Guduguntla, S.; Gualtierotti, J.; Goh, S.; Feringa, B. Enantioselective Synthesis of Di- and Tri-Arylated All-Carbon Quaternary Stereocenters via Copper-Catalyzed Allylic Arylations with Organolithium Compounds. ACS Catal. 2016, 6, 6591–6595.
- 11(a) Piarulli, U.; Daubos, P.; Claverie, C.; Roux, M.; Gennari, C. A Catalytic and Enantioselective Desymmetrization of meso Cyclic Allylic Bisdiethylphosphates with Organozinc Reagents. Angew. Chem. Int. Ed. 2003, 42, 234–236; (b) Jacques, R.; Pullin, R.; Fletcher, S. Desymmetrization of meso-Bisphosphates Using Copper Catalysis and Alkylzirconocene Nucleophiles. Nat. Commun. 2019, 10, 21; (c) Goh, S.; Guduguntla, S.; Kikuchi, T.; Lutz, M.; Otten, E.; Fujita, M.; Feringa, B. Desymmetrization of meso-Dibromocycloalkenes through Copper(I)-Catalyzed Asymmetric Allylic Substitution with Organolithium Reagents. J. Am. Chem. Soc. 2018, 140, 7052–7055.
- 12(a) Giannerini, M.; Fananas-Mastral, M.; Feringa, B. Z-Selective Copper-Catalyzed Asymmetric Allylic Alkylation with Grignard Reagents. J. Am. Chem. Soc. 2012, 134, 4108–4111; (b) Li, H.; Muller, D.; Guenee, L.; Alexakis, A. Copper-Catalyzed Enantioselective Synthesis of Axially Chiral Allenes. Org. Lett. 2012, 14, 5880–5883; (c) Chaves-Pouso, A.; Alvarez-Constantino, A.; Fananas-Mastral, M. Enantio- and Diastereoselective Copper-Catalyzed Allylboration of Alkynes with Allylic gem-Dichlorides. Angew. Chem. Int. Ed. 2022, 61, e202117696.
- 13For diastereoselective examples, see: (a) Narumi, T.; Kobayakawa, T.; Aikawa, H.; Seike, S.; Tamamura, H. Stereoselective Formation of Trisubstituted (Z)-Chloroalkenes Adjacent to a Tertiary Carbon Stereogenic Center by Organocuprate-Mediated Reduction/Alkylation. Org. Lett. 2012, 14, 4490–4493; (b) Kobayakawa, T.; Narumi, T.; Tamamura, H. Remote Stereoinduction in the Organocuprate-Mediated Allylic Alkylation of Allylic gem-Dichlorides: Highly Diastereoselective Synthesis of (Z)-Chloroalkene Dipeptide Isosteres. Org. Lett. 2015, 17, 2302–2305.
- 14For 4-halocrotonate derivatives, see: (a) Murphy, K.; Hoveyda, A. Enantioselective Synthesis of α-Alkyl-β,γ-unsaturated Esters through Efficient Cu-Catalyzed Allylic Alkylations. J. Am. Chem. Soc. 2003, 125, 4690–4691; (b) Lee, Y.; Hoveyda, A. Lewis Base Activation of Grignard Reagents with N-Heterocyclic Carbenes. Cu-Free Catalytic Enantioselective Additions to γ-Chloro-α,β-Unsaturated Esters. J. Am. Chem. Soc. 2006, 128, 15604–15605; (c) Hartog, T.; Maciá, B.; Minnaard, A.; Feringa, B. Copper-Catalyzed Asymmetric Allylic Alkylation of Halocrotonates: Efficient Synthesis of Versatile Chiral Multifunctional Building Blocks. Adv. Synth. Catal. 2010, 352, 999–1013.
- 15 Danheiser, R. 3-Butylcyclobutenone. Org. Synth. 1990, 68, 32.
- 16(a) Chen, P.; Dong, G. Cyclobutenones and Benzocyclobutenones: Versatile Synthons in Organic Synthesis. Chem. Eur. J. 2016, 22, 18290–18315; (b) Chen, J.; Zhou, Q.; Fang, H.; Lu, P. Chin. J. Chem. 2022, 40, 1346–1358.
- 17(a) Clement, H.; Boghi, M.; McDonald, R.; Bernier, L.; Coe, J.; Farrell, W.; Helal, C.; Reese, M.; Sach, N.; Lee, J.; Hall, D. High-Throughput Ligand Screening Enables the Enantioselective Conjugate Borylation of Cyclobutenones to Access Synthetically Versatile Tertiary Cyclobutylboronates. Angew. Chem. Int. Ed. 2019, 58, 18405–18409; (b) Zhong, C.; Huang, Y.; Zhang, H.; Zhou, Q.; Liu, Y.; Lu, P. Enantioselective Synthesis of 3-Substituted Cyclobutenes by Catalytic Conjugate Addition/Trapping Strategies. Angew. Chem. Int. Ed. 2020, 59, 2750–2754; (c) Cui, M.; Oestreich, M. Synthesis of Silylated Cyclobutanone and Cyclobutene Derivatives Involving 1,4-Addition of Zinc-Based Silicon Nucleophiles. Chem. Eur. J. 2021, 27, 16103–16106; (d) Yan, P.; Zhong, C.; Zhang, J.; Liu, Y.; Fang, H.; Lu, P. 3-(Methoxycarbonyl)Cyclobutenone as a Reactive Dienophile in Enantioselective Diels–Alder Reactions Catalyzed by Chiral Oxazaborolidinium Ions. Angew. Chem. Int. Ed. 2021, 60, 4609–4613; (e) Yan, P.; Zhang, J.; Lu, L.; Fang, H.; Lu, P. Enantioselective Construction of Vicinal Angular Quaternary Stereocenters Enabled by Strained Cyclobutenones. ACS Catal. 2022, 12, 15416–15423; (f) Wang, S.; Zhong, C.; Huang, Y.; Lu, P. Enantioselective Hydrofunctionalization of Cyclobutenones: Total Synthesis of gem-Dimethylcyclobutane Natural Products. Angew. Chem. Int. Ed. 2024, 63, e202400515; (g) Egea-Arrebola, D.; Goetzke, F.; Fletcher, S. Rhodium-Catalyzed Asymmetric Arylation of Cyclobutenone Ketals. Angew. Chem. Int. Ed. 2023, 62, e202217381.
- 18(a) Dillon, J.; Gao, Q. Reactions of Dichlorocyclobutenones with nucleophiles: A Synthesis of Some New Cyclobutenones and an Unusual Ring Expansion to a Butenolide. J. Org. Chem. 1994, 59, 6868–6870; (b) Yan, M.; Zhou, Q.; Lu, P. Collective Synthesis of Chiral Tetrasubstituted Cyclobutanes Enabled by Enantioconvergent Negishi Cross-Coupling of Cyclobutenones. Angew. Chem. Int. Ed. 2023, 62, e202218008. For a recent example with dichlorocyclobutanone, see: (c) Wang, M.; Wang Z.; Lu, P. Modular Synthesis of Multi-substituted Cyclobutanones Enabled by Oxyallyl Cations. Chin. J. Chem. 2024, 42, 459–463.
- 19 Yan, P.; Zhou, Q. Chen, J.; Lu, P. Controllable Skeleton Rearrangement of 3-Substituted Cyclobutanones under Basic Conditions. Chin. J. Chem. 2020, 38, 1103–1106.
- 20(a) Salvi, L.; Kim, J.; Walsh, P. Practical Catalytic Asymmetric Synthesis of Diaryl-, Aryl Heteroaryl-, and Diheteroarylmethanols. J. Am. Chem. Soc. 2009, 131, 12483–12493; (b) Hevia, E.; Mulvey, R. Split Personality of Lithium Chloride: Recent Salt Effects in Organometallic Recipes. Angew. Chem. Int. Ed. 2011, 50, 6448–6450.
- 21 Shintani, R.; Tokunaga, N.; Doi, H.; Hayashi, T. A New Entry of Nucleophiles in Rhodium-Catalyzed Asymmetric 1,4-Addition Reactions: Addition of Organozinc Reagents for the Synthesis of 2-Aryl-4-piperidones. J. Am. Chem. Soc. 2004, 126, 6240–6241.
- 22(a) Shintani, R.; Duan, W.; Hayashi, T. Rhodium-Catalyzed Asymmetric Construction of Quaternary Carbon Stereocenters: Ligand-Dependent Regiocontrol in the 1,4-Addition to Substituted Maleimides. J. Am. Chem. Soc. 2006, 128, 5628–5629; (b) Holder, J.; Zou, L.; Marziale, A.; Liu, P.; Lan, Y.; Gatti, M.; Kikushima, K.; Houk, K.; Stoltz, B. Mechanism and Enantioselectivity in Palladium-Catalyzed Conjugate Addition of Arylboronic Acids to β-Substituted Cyclic Enones: Insights from Computation and Experiment. J. Am. Chem. Soc. 2013, 135, 14996–15007.
- 23(a) Glynn, D.; Shannon, J.; Woodward, S. On the Scope of Trimethylaluminium-Promoted 1,2-Additions of ArZnX Reagents to Aldehydes. Chem. Eur. J. 2010, 16, 1053–1060; (b) Shannon, J.; Bernier, D.; Rawson, D.; Woodward, S. Direct Asymmetric Catalytic 1,2-Addition of RZnX to Aldehydes Promoted by AlMe3 and Reversal of Expected Stereochemistry. Chem. Commun. 2007, 3945–3947; (c) Goldsmith, P.; Teat, S.; Woodward, S. Enantioselective Preparation of β,β-Disubstituted α-Methylenepropionates by MAO Promotion of the Zinc Schlenk Equilibrium. Angew. Chem. Int. Ed. 2005, 44, 2235–2237.
- 24Deposition numbers 2350900 (for 8b), 2350901 (for 12), 2352488 (for epi-13-imide), 2350902 (for 15), and 2350903 (for 18) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 25 Torii, S.; Inokuchi, T.; Kondo, K. A Facile Procedure for Oxidative Cleavage of Enolic Olefins to the Carbonyl Compounds with Ruthenium Tetroxide (RuO4). J. Org. Chem. 1985, 50, 4980–4982.
- 26 Luche, J. Lanthanides in organic chemistry. 1. Selective 1,2 reductions of conjugated ketones. J. Am. Chem. Soc. 1978, 100, 2226–2227.
- 27Similar rapid epimerization was observed, see: Chang, S.; Holmes, M.; Mowat, J.; Meanwell, M.; Britton, R. α-Arylation and Ring Expansion of Annulated Cyclobutanones: Stereoselective Synthesis of Functionalized Tetralones. Angew. Chem. Int. Ed. 2017, 56, 748–752.




