Photolabile ortho-Nitro-Benzyl Carbonate as a Permanent Hydroxyl Protecting Group for the Synthesis of Digalactosyl Diacylglycerol
Jibin Zheng
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorHongyu Chen
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorJintao Shang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorLvfeng Zhang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorYouling Liang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorDongsheng Chang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
You Yang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Engineering Research Center of Pharmaceutical Process Chemistry, Ministry of Education, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorJibin Zheng
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorHongyu Chen
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorJintao Shang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorLvfeng Zhang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorYouling Liang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorDongsheng Chang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
You Yang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Engineering Research Center of Pharmaceutical Process Chemistry, Ministry of Education, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
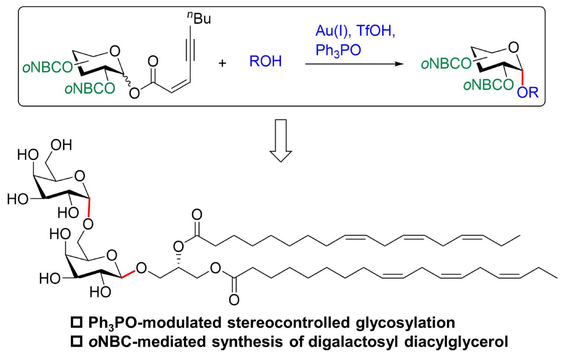
Traditional protecting groups are often removed under harsh conditions with potentially hazardous reagents, thereby impeding the convenient synthesis of oligosaccharides and glycosides. Herein, we present to utilize the photolabile ortho-nitro-benzyl carbonate (oNBC) as a permanent hydroxyl protecting group for stereocontrolled synthesis of glycosides. The Ph3PO-modulated glycosylation with strongly disarmed per-O-oNBC-protected glycosyl ynenoates preferred to afford glycosides with excellent α-selectivities via the β-phosphonium transition state. Based on the oNBC-mediated galactosylation, synthesis of the glycolipid digalactosyl diacylglycerol (DGDG) containing six double bonds and two esters was achieved in a straightforward manner.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400866-sup-0001-supinfo.pdfPDF document, 8.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 3rd ed., Wiley, New York, 1999;
10.1002/0471220574 Google Scholar(b) Fraser-Reid, B.; Jayaprakash, K. N.; Lopez, J. C.; Gomez, A. M.; Uriel, C. Protecting Groups in Carbohydrate Chemistry Profoundly Influence All Selectivities in Glycosyl Couplings. In Frontiers in Modern Carbohydrate Chemistry, ACS Symposium Series 960, American Chemical Society, Washington, DC, 2007, pp. 91–117.10.1021/bk-2007-0960.ch007 Google Scholar
- 2(a) Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C. “One-Pot” Protection, Glycosylation, and Protection-Glycosylation Strategies of Carbohydrates. Chem. Rev. 2018, 118, 8025−8104; (b) Nielsen, M. M.; Pedersen, C. M. Catalytic Glycosylations in Oligosaccharide Synthesis. Chem. Rev. 2018, 118, 8285−8358.
- 3(a) Guberman, M.; Seeberger, P. H. Automated Glycan Assembly: A Perspective. J. Am. Chem. Soc. 2019, 141, 5581−5592; (b) Li, X.; Yang, Y. Automated Chemical Solid-Phase Synthesis of Glycans. Chin. J. Chem. 2022, 40, 1714−1728.
- 4 Wang, T.; Demchenko, A. V. Synthesis of Carbohydrate Building Blocks via Regioselective Uniform Protection/Deprotection Strategies. Org. Biomol. Chem. 2019, 17, 4934−4950.
- 5 Crawford, C.; Oscarson, S. Optimized Conditions for the Palladium- Catalyzed Hydrogenolysis of Benzyl and Naphthylmethyl Ethers: Preventing Saturation of Aromatic Protecting Groups. Eur. J. Org. Chem. 2020, 2020, 3332−3337.
- 6 Guberman, M.; Brautigam, M.; Seeberger, P. H. Automated Glycan Assembly of Lewis Type I and II Oligosaccharide Antigens. Chem. Sci. 2019, 10, 5634−5640.
- 7(a) Adinolfi, M.; Barone, G.; Guariniello, L.; Iadonisi, A. Facile Cleavage of Carbohydrate Benzyl Ethers and Benzylidene Acetals Using the NaBrO3/Na2S2O4 Reagent under Two-Phase Conditions. Tetrahedron Lett. 1999, 40, 8439−8441; (b) Niemietz, M.; Perkams, L.; Hoffman, J.; Eller, S.; Unverzagt, C. Selective Oxidative Debenzylation of Mono- and Oligosaccharides in the Presence of Azides. Chem. Commun. 2011, 47, 10485–10487.
- 8 Yang, Y.; Laval, S.; Yu, B. Chemical Synthesis of Saponins. Adv. Carbohydr. Chem. Biochem. 2014, 71, 137–226.
- 9(a) Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of Digalactosyl Diacylglycerols and Their Structure–Inhibitory Activity on Human Lanosterol Synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159; (b) Mullapud, V. B.; Craig, K. C.; Guo, Z. Synthesis of a Bifunctionalized Glycosylphosphatidylinositol (GPI) Anchor Useful for the Study of GPI Biology. Chem. Eur. J. 2023, 29, e2022034.
- 10 Inuki, S.; Kishi, J.; Kashiwabara, E.; Aiba, T.; Fujimoto, Y. Convergent Synthesis of Digalactosyl Diacylglycerols. Org. Lett. 2017, 19, 6482−6485.
- 11(a) Klán, P.; Šolomek, T.; Bochet, C. G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191; (b) Yu, H.; Li, J.; Wu, D.; Qiu, Z.; Zhang, Y. Chemistry and Biological Applications of Photo-Labile Organic Molecules. Chem. Soc. Rev. 2010, 39, 464–473.
- 12 Wang, P. F. Photolabile Protecting Groups: Structure and Reactivity. Asian J. Org. Chem. 2013, 2, 452–464.
- 13(a) Buda, S.; Golebiowska, P.; Mlynarski, J. Application of the 2-Nitrobenzyl Group in Glycosylation Reactions: A Valuable Example of an Arming Participating Group. Eur. J. Org. Chem. 2013, 2013, 3988–3991; (b) Tiwari, V.; Singh, A. K.; Chaudhary, P.; Seeberger, P. H.; Kandasamy, J. Synthesis of Photolabile Protecting Group (PPG) Protected Uronic Acid Building Blocks: Applications in Carbohydrate Synthesis with the Assistance of a Continuous Flow Photoreactor. Org. Chem. Front. 2019, 6, 3859–3863; (c) Wang, J.; Feng, Y.; Sun, T.; Zhang, Q.; Chai, Y. Photolabile 2-(2-Nitrophenyl)-propyloxycarbonyl (NPPOC) for Stereoselective Glycosylation and Its Application in Consecutive Assembly of Oligosaccharides. J. Org. Chem. 2022, 87, 3402−3421; (d) Hu, C.; Wu, S.; He, F.; Cai, D.; Xu, Z.; Ma, W.; Liu, Y.; Wei, B.; Li, T.; Ding, K. Convergent Synthesis and Anti-Pancreatic Cancer Cell Growth Activity of a Highly Branched Heptadecasaccharide from Carthamus tinctorius. Angew. Chem. Int. Ed. 2022, 61, e202202554.
- 14 Cavedon, C.; Sletten, E. T.; Madani, A.; Niemeyer, O.; Seeberger, P. H. Visible-Light-Mediated Oxidative Debenzylation Enables the Use of Benzyl Ethers as Temporary Protecting Groups. Org. Lett. 2021, 23, 514−518.
- 15 Samarasimhareddy, M.; Alshanski, I.; Mervinetsky, E.; Hurevich, M. Photodeprotection of up to Eight Photolabile Protecting Groups from a Single Glycan. Synlett 2018, 29, 880–884.
- 16 Li, X.; Ma, Z.; Liu, R.; Hurevich, M.; Yang, Y. Photolabile protecting group-mediated synthesis of 2-deoxy-glycosides. Chin. J. Chem. 2021, 39, 3309–3314.
- 17(a) Mootoo, D. R.; Konradsson, P.; Udodong, U.; Fraser-Reid, B. “Armed” and “Disarmed” n-Pentenyl Glycosides in Saccharide Couplings Leading to Oligosaccharides. J. Am. Chem. Soc. 1988, 110, 5583−5584; (b) Hsu, C-H.; Hung, S.-C.; Wu, C.-Y.; Wong, C.-H. Toward Automated Oligosaccharide Synthesis. Angew. Chem. Int. Ed. 2011, 50, 11872–11923.
- 18(a) Li, X.; Di Carluccio, C.; Miao, H.; Zhang, L.; Shang, J.; Molinaro, A.; Xu, P.; Silipo, A.; Yu, B.; Yang, Y. Promoter-Controlled Synthesis and Conformational Analysis of Cyclic Mannosides up to a 32-mer. Angew. Chem. Int. Ed. 2023, 62, e202307851; (b) Li, X.; Li, C.; Liu, R.; Wang, J.; Wang, Z.; Chen, Y.; Yang, Y. Gold(I)-Catalyzed Glycosylation with Glycosyl Ynenoates as Donors. Org. Lett. 2019, 21, 9693−9698; (c) Liu, R.; Chen, Y.; Zheng, J.; Zhang, L.; Xu, T.; Xu, P.; Yang, Y. Synthesis of Nucleosides and Deoxynucleosides via Gold(I)-Catalyzed N-Glycosylation of Glycosyl (Z)-Ynenoates. Org. Lett. 2022, 24, 9479−9484; (d) Zhang, Y.; Wang, X.; Liang, Y.; Zhang, L.; Fan, J.; Yang, Y. A Semisynthetic Oligomannuronic Acid-Based Glycoconjugate Vaccine against Pseudomonas aeruginosa. ACS Cent. Sci. 2024, 10, 1515–1523.
- 19(a) Lu, S.-R.; Lai, Y.-H.; Chen, J.-H.; Liu, C.-Y.; Mong, K.-K. T. Dimethylformamide: An Unusual Glycosylation Modulator. Angew. Chem. Int. Ed. 2011, 50, 7315−7320; (b) Lou, Q.; Hua, Q.; Zhang, L.; Yang, Y. Dimethylformamide-Modulated Kdo Glycosylation for Stereoselective Synthesis of α-Kdo Glycosides. Org. Lett. 2020, 22, 981–985; (c) Wei, R.; Liu, H.; Tang, A. H.; Payne, R. J.; Li, X. A Solution to Chemical Pseudaminylation via a Bimodal Glycosyl Donor for Highly Stereocontrolled α- and β-Glycosylation. Org. Lett. 2019, 21, 3584−3588.
- 20(a) Mukaiyama, T.; Kobashi, Y. Highly α-Selective Synthesis of Disaccharide Using Glycosyl Bromide by the Promotion of Phosphine Oxide. Chem. Lett. 2004, 33, 10–11; (b) Wang, L.; Overkleeft, H. S.; van der Marel, G. A.; Codee, J. D. C. Reagent Controlled Stereoselective Synthesis of α-Glucans. J. Am. Chem. Soc. 2018, 140, 4632−4638; (c) Zeng, J.; Wang, R.; Zhang, S.; Fang, J.; Liu, S.; Sun, G.; Xu, B.; Xiao, Y.; Fu, D.; Zhang, W.; Hu, Y.; Wan, Q. Hydrogen-Bonding-Assisted Exogenous Nucleophilic Reagent Effect for β-Selective Glycosylation of Rare 3-Amino Sugars. J. Am. Chem. Soc. 2019, 141, 8509−8515; (d) Zhang, Y.; He, H.; Chen, Z.; Huang, Y.; Xiang, G.; Li, P.; Yang, X.; Lu, G.; Xiao, G. Merging Reagent Modulation and Remote Anchimeric Assistance for Glycosylation: Highly Stereoselective Synthesis of α-Glycans up to a 30-mer. Angew. Chem. Int. Ed. 2021, 60, 12597−12606.
- 21(a) Li, Y.; Yang, Y.; Yu, B. An Efficient Glycosylation Protocol with Glycosyl ortho-Alkynylbenzoates as Donors Under the Catalysis of Ph3PAuOTf. Tetrahedron Lett. 2008, 49, 3604−3608; (b) Tang, Y.; Li, J.; Zhu, Y.; Li, Y.; Yu, B. Mechanistic Insights into the Gold(I)-Catalyzed Activation of Glycosyl ortho-Alkynylbenzoates for Glycosidation. J. Am. Chem. Soc. 2013, 135, 18396−18405; (c) Yu, B. Gold(I)-Catalyzed Glycosylation with Glycosyl o-Alkynylbenzoates as Donors. Acc. Chem. Res. 2018, 51, 507−516.
- 22(a) Hölzl, G.; Dörmann, P. Structure and Function of Glycoglycerolipids in Plants and Bacteria. Prog. Lipid Res. 2007, 46, 225−243;
(b) Zhang, J.; Li, C.; Yu, G.; Guan, H. Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms. Mar. Drugs 2014, 12, 3634−3659;
(c) Yagami, N.; Vibhute, A. M.; Tanaka, H.-N.; Komura, N.; Imamura, A.; Ishida, H.; Ando, H. Stereoselective Synthesis of Diglycosyl Diacylglycerols with Glycosyl Donors Bearing a β-Stereodirecting 2,3-Naphthalenedimethyl Protecting Group. J. Org. Chem. 2020, 85, 6166–16181.
10.1021/acs.joc.0c02121 Google Scholar
- 23 van der Vorm, S.; Hansen, T.; Overkleeft, H. S.; van der Marel, G. A.; Codee, J. D. C. The Influence of Acceptor Nucleophilicity on the Glycosylation Reaction Mechanism. Chem. Sci. 2017, 8, 1867–1875
- 24 Hsu, M.-Y.; Liu, Y.-P.; Lam, S.; Lin, S.-C.; Wang, C.-C. TMSBr-Mediated Solvent- and Work-Up-Free Synthesis of α-2-Deoxyglycosides from Glycals. Beilstein J. Org. Chem. 2016, 12, 1758–1764.




