Palladium Complexes with N,O-Bidentate Ligands Based on N-Oxide Units from Cyclic Secondary Amines: Synthesis and Catalytic Application in Mizoroki-Heck Reaction
Corresponding Author
Xuefeng Jia
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYanyan Wen
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
These authors contributed equally to this work.
Search for more papers by this authorChanghui He
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Xianqiang Huang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xuefeng Jia
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYanyan Wen
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
These authors contributed equally to this work.
Search for more papers by this authorChanghui He
Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education, School of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, Shanxi, 030032 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Xianqiang Huang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Palladium-catalyzed Mizoroki-Heck reaction is a powerful and efficient method for construction of Csp2–Csp2 bonds. Herein, four palladium complexes (I—IV) with N,O-bidentate ligands (L1—L4) based on N-oxide units from cyclic secondary amines were easily synthesized and successfully applied in Mizoroki-Heck reaction of aryl bromides with electron-deficient olefins. X-ray diffraction analyses indicated the palladium(II) atom of II took the distorted square planar geometry and was four-coordinated by nitrogen and oxygen atoms from two ligands (L2). Two free chloride ions were presented as counter anions in complex II. But the palladium(II) center of IV was coordinated by nitrogen and oxygen atoms from one ligand (L4) as well as two chlorine atoms, which exhibited the nearly square-planar geometry. The study on catalytic properties of palladium complexes revealed that complex II exhibited high activity superior to the other complexes. The coupling reactions of a series of aryl bromides and olefin derivatives proceeded in the presence of 2—5 mol% palladium complex II, giving the desired products in good to excellent yields. The advantages of this method such as good compatibility of functional groups, high yields, and short reaction times made it more attractive for constructing Csp2–Csp2 bonds in the synthesis of functional molecules and materials.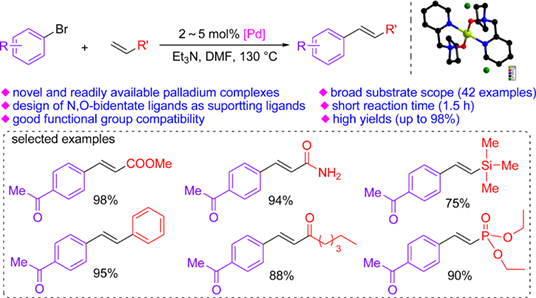
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300375-sup-0001-supinfo.pdfPDF document, 3.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Beletskaya, I. P.; Cheprakov, A. V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066; (b) Heravi, M.; Hashemi, E.; Ghobadi, N. Development of Recent Total Syntheses Based on the Heck Reaction. Curr. Org. Chem. 2013, 17, 2192–2224; (c) Beletskaya, I. P.; Cheprakov, A. V. Modern Heck Reactions. In New Trends in Cross-Coupling: Theory and Applications, Ed.: Colacot, T., The Royal Society of Chemistry, Cambridge, U.K., 2015, pp. 335–478.
- 2(a) Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043; (b) Vries, J. G. Palladium-Catalysed Coupling Reactions. In Organometallics as Catalysts in the Fine Chemical Industry, Eds.: Beller, M.; Blaser, H.-U., Springer Berlin, Heidelberg, 2012, pp. 1–34.
- 3(a) Dounay, A. B.; Overman, L. E. The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev. 2003, 103, 2945–2964; (b) Tietze, L. F.; Düfert, A. Multiple Pd-catalyzed reactions in the synthesis of natural products, drugs, and materials. Pure Appl. Chem. 2010, 82, 1375–1392; (c) Czajkowska-Szczykowska, D.; Morzycki, J. W.; Wojtkielewicz, A. Pd-catalyzed steroid reactions. Steroids 2015, 97, 13–44; (d) Watanabe, S.; Nishikawa, T.; Nakazaki, A. Synthesis of Oxy-Functionalized Steroidal Skeletons via Mizoroki-Heck and Intramolecular Diels-Alder Reactions. Org. Lett. 2019, 21, 7410–7414.
- 4(a) Prashad, M. Palladium-catalyzed Heck arylations in the synthesis of active pharmaceutical ingredients. Top. Organomet. Chem. 2004, 6, 181–203; (b) Tietze, L. F.; Kinzel, T. Synthesis of natural products and analogs using multiple Pd-catalyzed transformations. Pure Appl. Chem. 2007, 79, 629–650; (c) Kotora, M.; Hessler, F.; Eignerová, B. Transition-Metal-Mediated or -Catalyzed Syntheses of Steroids and Steroid-Like Compounds. Eur. J. Org. Chem. 2012, 29–42.
- 5(a) Schiedel, M.-S.; Briehn, C. A.; Bauerle, P. C-C Cross-coupling reactions for the combinatorial synthesis of novel organic materials. J. Organomet. Chem. 2002, 653, 200–208; (b) Hang, C.; Wu, H.-W.; Zhu, L.-L. π-Conjugated cyanostilbene cyanostilbene-based optoelectric functional materials. Chin. Chem. Lett. 2016, 27, 1155–1165.
- 6(a) Martinez, R.; Voica, F.; Genet, J.-P.; Darses, S. Base-Free Mizoroki-Heck Reaction Catalyzed by Rhodium Complexes. Org. Lett. 2007, 9, 3213–3216; (b) Tanaka, S.; Itami, K.; Sunahara, K.; Tatsuta, G.; Mori, A. Ethylaluminum as an ethylene source for the Mizoroki-Heck- type reaction. Rhodium-catalyzed preparation of stilbene derivatives. Chem. Commun. 2015, 51, 1949–1952.
- 7Oi, S.; Sakai, K.; Inoue, Y. Ruthenium-Catalyzed Arylation of 2-Alkenylpyridines with Aryl Bromides: Alternative E,Z-Selectivity to Mizoroki-Heck Reaction. Org. Lett. 2005, 7, 4009–4011.
- 8(a) Wang, S.-S.; Yang, G.-Y. Recent developments in low-cost TM-catalyzed Heck-type reactions (TM = transition metal, Ni, Co, Cu, and Fe). Catal. Sci. Technol. 2016, 6, 2862–2876; (b) Gøgsig, T. M.; Kleimark, J.; Nilsson Lill, S. O.; Korsager, S.; Lindhardt, A. T.; Norrby, P. O.; Skrydstrup, T. Mild and Efficient Nickel-Catalyzed Heck Reactions with Electron-Rich Olefins. J. Am. Chem. Soc. 2012, 134, 443–452; (c) Standley, E. A.; Jamison, T. F. Simplifying Nickel(0) Catalysis: An Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes. J. Am. Chem. Soc. 2013, 135, 1585–1592; (d) Walker, B. R.; Sevov, C. S. An Electrochemically Promoted, Nickel-Catalyzed Mizoroki-Heck Reaction. ACS Catal. 2019, 9, 7197–7203; (e) Nishikata, T.; Noda, Y.; Fujimoto, R.; Sakashita, T. An Efficient Generation of a Functionalized Tertiary-Alkyl Radical for Copper-catalyzed Tertiary-Alkylative Mizoroki-Heck type Reaction. J. Am. Chem. Soc. 2013, 135, 16372–16375; (f) Xiong, H.; Li, Y.; Qian, B.; Wei, R.; Van der Eycken, E. V.; Bao, H. Iron(II)-Catalyzed Heck-Type Coupling of Vinylarenes with Alkyl Iodides. Org. Lett. 2019, 21, 776–779.
- 9(a) Biffis, A.; Centomo, P.; Zotto, A. D.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295; For selected examples on palladium-catalyzed Heck reactions, see: (b) Carrow, B. P.; Hartwig, J. F. Ligandless, Anionic, Arylpalladium Halide Intermediates in the Heck Reaction. J. Am. Chem. Soc. 2010, 132, 79–81; (c) Werner, E. W.; Sigman, M. S. Operationally Simple and Highly (E)-Styrenyl- Selective Heck Reactions of Electronically Nonbiased Olefins. J. Am. Chem. Soc. 2011, 133, 9692–9695; (d) Schroeter, F.; Strassner, T. Understanding Anionic “Ligandless” Palladium Species in the Mizoroki-Heck Reaction. Inorg. Chem. 2018, 57, 5159–5173; (e) Li, C.; Qiang, X.-Y.; Qi, Z.-C.; Cao, B.; Li, J.-Y.; Yang, S.-D. Pd-Catalyzed Heck-Type Reaction: Synthesizing Highly Diastereoselective and Multiple Aryl-Substituted P-Ligands. Org. Lett. 2019, 21, 7138–7142; (f) Yang, Q.; Sane, N.; Klosowski, D.; Lee, M.; Rosenthal, T.; Wang, N. X.; Wiensch, E. Mizoroki-Heck Cross-Coupling of Bromobenzenes with Styrenes: Another Example of Pd-Catalyzed Cross-Coupling with Potential Safety Hazards. Org. Process Res. Dev. 2019, 23, 2148–2156; (g) Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B. H. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki-Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382; (h) Li, Y.; Hao, M.; Chang, Y.-C.; Liu, Y.; Wang, W.-F.; Sun, N,; Zhu, W.-Q.; Gao, Z. Synthesis of 4-trifluoromethylated 1,3-butadienes via Palladium catalyzed Heck reaction. Chin. J. Chem. 2021, 39, 2962–2966; (i) Valentini, F.; Carpisassi, L.; Comѐs, A.; Aprile, C.; Vaccaro, L. Tailor-Made POLITAG-Pd0 Catalyst for the Low-Loading Mizoroki-Heck Reaction in γ-Valerolactone as a Safe Reaction Medium. ACS Sustainable Chem. Eng. 2022, 10, 12386–12393.
- 10(a) Yang, Z.; Zhou, J. (Steve) Palladium-Catalyzed, Asymmetric Mizoroki-Heck Reaction of Benzylic Electrophiles Using Phosphoramidites as Chiral Ligands. J. Am. Chem. Soc. 2012, 134, 11833–11835; (b) Tay, D. W.; Jong, H.; Lim, Y. H.; Wu, W.; Chew, X.; Robins, E. G.; Johannes, C. W. Palladium-meta-Terarylphosphine Catalyst for the Mizoroki-Heck Reaction of (Hetero)Aryl Bromides and Functional Olefins. J. Org. Chem. 2015, 80, 4054–4063; (c) Lee, J.-Y.; Shen, J.-S.; Tzeng, R.-J.; Lu, I. C.; Li, J.-H.; Hu, C.-H.; Lee, H. M. Well-defined palladium(0) complexes bearing N-heterocyclic carbene and phosphine moieties: efficient catalytic applications in the Mizoroki-Heck reaction and direct C−H functionalization. Dalton Trans. 2016, 45, 10375–10388.
- 11(a) Fortman, G. C.; Nolan, S. P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169; (b) Lebel, H.; Janes, M. K.; Charette, A. B.; Nolan, S. P. Structure and Reactivity of ″Unusual″ N-Heterocyclic Carbene (NHC) Palladium Complexes Synthesized from Imidazolium Salts. J. Am. Chem. Soc. 2004, 126, 5046–5047; (c) Hsu, Y. C.; Shen, J. S.; Lin, B. C.; Chen, W. C.; Chan, Y. T.; Ching, W. M.; Yap, G. P. A.; Hsu, C. P.; Ong, T. G. Synthesis andisolation of an acyclic tridentate bis(pyridine)carbodicarbene and studies on its structural implications and reactivities. Angew. Chem. Int. Ed. 2015, 54, 2420–2424; (d) Guest, D.; Silva, V. H. M.; Batista, A. P. L.; Roe, S. M.; Braga, A. A. C.; Navarro, O. (N-Heterocyclic Carbene)-Palladate Complexes in Anionic Mizoroki-Heck Coupling Cycles: A Combined Experimental and Computational Study. Organometallics 2015, 34, 2463–2470; (e) Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. An efficient class of bis-NHC salts: Applications in Pd-catalyzed reactions under mild reaction conditions. RSC Adv. 2018, 8, 26407–26415.
- 12(a) Mino, T.; Shirae, Y.; Sasai, Y.; Sakamoto, M.; Fujita, T. Phosphine- Free Palladium Catalyzed Mizoroki-Heck Reaction Using Hydrazone as a Ligand. J. Org. Chem. 2006, 71, 6834–6839; (b) Haneda, S.; Gan, Z.; Eda, K.; Hayashi, M. Ligand Effects of 2-(2-Pyridyl)benzazole-Pd Complexes on the X-ray Crystallographic Structures, 1H NMR Spectra, and Catalytic Activities in Mizoroki-Heck Reactions. Organometallics 2007, 26, 6551–6555; (c) Boyd, P. D. W.; Wright, L. J.; Zafar, M. N. Extending the Range of Neutral N-Donor Ligands Available for Metal Catalysts: N-[1-Alkylpyridin-4(1H)-ylidene]amides in Palladium-Catalyzed Cross-Coupling Reactions. Inorg. Chem. 2011, 50, 10522–10524; (d) Suzaki, Y.; Shimada, K.; Chihara, E.; Saito, T.; Tsuchido, Y.; Osakada, K. Rotaxane-Based Dinuclear Palladium Catalysts for Ring-closure Mizoroki-Heck Reaction. Org. Lett. 2011, 13, 3774–3777; (e) Yang, L.; Zhang, X.; Mao, P.; Xiao, Y.; Bian, H.; Yuan, J.; Mai, W.; Qu, L. NCN pincer palladium complexes based on 1,3-dipicolyl-3,4,5,6-tetrahydropyrimidin-2-ylidenes: synthesis, characterization and catalytic activities. RSC Adv. 2015, 5, 25723–25729.
- 13Liu, Q.-X.; Hu, Z.-L.; Yu, S.-C.; Zhao, Z.-X.; Wei, D.-C.; Li, H.-L. NHC Pd(II) and Ag(I) Complexes: Synthesis, Structure, and Catalytic Activity in Three Types of C−C Coupling Reactions. ACS Omega 2018, 3, 4035–4047.
- 14Ortiz, A.; Gómez-Sal, P.; Flores, J. C.; de Jesús, E. Highly Recoverable Pd(II) Catalysts for the Mizoroki-Heck Reaction Based on N-Heterocyclic Carbenes and Poly(benzylether) Dendrons. Organometallics 2018, 37, 3598–3610.
- 15Sharma, K. N.; Satrawala, N.; Srivastava, A. K.; Ali, M.; Joshi, R. K. Palladium(II) ligated with a selenated (Se, CNHC, N−)-type pincer ligand: an efficient catalyst for Mizoroki-Heck and Suzuki-Miyaura coupling in water. Org. Biomol. Chem. 2019, 17, 8969–8976.
- 16Zhu, J.; Lindsay, V. N. G. Benzimidazolyl Palladium Complexes as Highly Active and General Bifunctional Catalysts in Sustainable Cross-Coupling Reactions. ACS Catal. 2019, 9, 6993–6998.
- 17(a) Lee, J.-Y.; Su, Y.-S.; Wang, Y.-S.; Lee, H. M. Tetranuclear Palladium Complexes of Abnormal N-Heterocyclic Carbene Ligands and Their Catalytic Activities in Mizoroki-Heck Coupling Reaction of Electron-Rich Aryl Chlorides. Adv. Synth. Catal. 2019, 361, 4714–4726; (b) Hung, C.-H.; Zheng, W.-Y.; Lee, H. M. Palladium Complexes with Phenoxy- and Amidate-Functionalized N-Heterocyclic Carbene Ligands Based on 3-Phenylimidazo[1,5-a]pyridine: Synthesis and Catalytic Application in Mizoroki-Heck Coupling Reactions with Ortho-Substituted Aryl Chlorides. Organometallics 2021, 40, 702–713.
- 18Chakrabortty, S.; Kaur, M.; Adhikari, M.; Manar, K. K.; Singh, S. A Bis (BICAAC) Palladium(II) Complex: Synthesis and Implementation as Catalyst in Heck-Mizoroki and Suzuki-Miyaura Cross Coupling Reactions. Inorg. Chem. 2021, 60, 6209–6217.
- 19Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496.
- 20Lo, C. H.; Lee, H. M. Synthesis and Characterization of C,C-Type Palladacycles and Their Catalytic Application in Mizoroki-Heck Coupling Reaction. Organometallics 2018, 37, 1150–1159.
- 21(a) Jia, X. F.; Zhao, F. Novel phosphorus-coordinated palladium(II) complexes derived from 3,5-disubstituted-1H-1,2,4-diazaphospholes: Synthesis and catalytic application in Suzuki-Miyaura cross-coupling reactions. Inorg. Chim. Acta 2017, 461, 145–149; (b) Zhao, F.; Xin, L.; Zhang, Y. Y.; Jia, X. F. Monodentate phosphorus-coordinated palladium(II) complexes as new catalyst for Mizoroki-Heck reaction of aryl halides with electron-deficient olefins. Chin. Chem. Lett. 2018, 29, 493–496.
- 22(a) Jia, X. F.; Peng, P.; Cui, J.; Xin, N. N.; Huang, X. Q. Four N,O-Bidentate Chelating Ligand-Tunable Copper(II) Complexes: Synthesis, Structural Characterization and Exceptional Catalytic Properties for Chan-Lam Coupling Reactions. Asian J. Org. Chem. 2018, 7, 1093–1100; (b) Jia, X. F.; Peng, P. N,O-Bidentate ligand-tunable copper(II) complexes as a catalyst for Chan-Lam coupling reactions of arylboronic acids with 1H-imidazole derivatives. Org. Biomol. Chem. 2018, 16, 8984–8988.
- 23Jia, X. F.; Peng, P. N-(4-Thiazolylmethyl)Morpholine N-Oxide as N,O-Bidentate Ligand for Copper-Catalyzed Ullmann-type N-Arylation of Azoles/Amines with Aryl Halides. Asian J. Org. Chem. 2019, 8, 1548–1554.
- 24(a) Jia, X. F.; Han, J. Rhodium-Catalyzed Direct C-H Amidation of Azobenzenes with Sulfonyl Azides: A Synthetic Route to Sterically Hindered ortho-Substituted Aromatic Azo Compounds. J. Org. Chem. 2014, 79, 4180–4185; (b) Han, J.; Pan, C. D.; Jia, X. F.; Zhu, C. J. Rhodium-catalyzed ortho-cyanation of symmetrical azobenzenes with N-cyano-N-phenyl-p-toluenesulfonamide. Org. Biomol. Chem. 2014, 12, 8603–8606; (c) Jia, X. F.; He, J. Three copper(II) complexes derived from 2-methylquinoline and cyclic secondary amines: Synthesis and catalytic application in C-N bond forming reactions. Appl. Organomet. Chem. 2022, 36, e6743.
- 25Boursalian, G. B.; Ngai, M.-Y.; Hojczyk, K. N.; Ritter, T. Pd-Catalyzed Aryl C-H Imidation with Arene as the Limiting Reagent. J. Am. Chem. Soc. 2013, 135, 13278–13281.




