Nucleophilic Radicals as Hydrogen Atom Abstractors in C(sp3)-H Functionalization Reactions
Comprehensive Summary
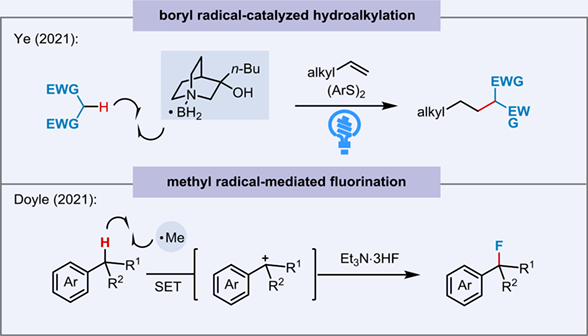
Hydrogen atom transfer (HAT) is an elementary mechanistic step in organic synthesis. The photoredox-catalyzed HAT has transformed organic synthesis by enabling the activation and subsequent cross-coupling of traditionally inert yet ubiquitous C(sp3)-H bonds. While diverse electrophilic radical species have proven to be exceptionally effective HAT catalysts for selective functionalization of hydridic and electron-neutral C(sp3)-H, general methods using nucleophilic radicals as hydrogen atom abstractors in C(sp3)-H functionalization remain relatively underdeveloped. Herein, we highlight two creative and strategic advances in addressing this long-standing challenge, and emphasize reaction achievements, mechanistic insights, potential, and future challenges.