Chemical Synthesis of an Octasaccharide Derivative Related to Group B Streptococcus Cell-Wall Polysaccharide
Chongzhen Sun
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorZhaojun Liu
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorWuxian Zeng
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorXiaolin Ma
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorGuirong Wang
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Guofeng Gu
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
NMPA Key Laboratory for Quality Research and Evaluation of Carbohydrate-based Medicine, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
E-mail: [email protected]Search for more papers by this authorChongzhen Sun
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorZhaojun Liu
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorWuxian Zeng
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorXiaolin Ma
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorGuirong Wang
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Guofeng Gu
National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
NMPA Key Laboratory for Quality Research and Evaluation of Carbohydrate-based Medicine, Shandong University, 72 Binhai Road, Qingdao, Shandong, 266237 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
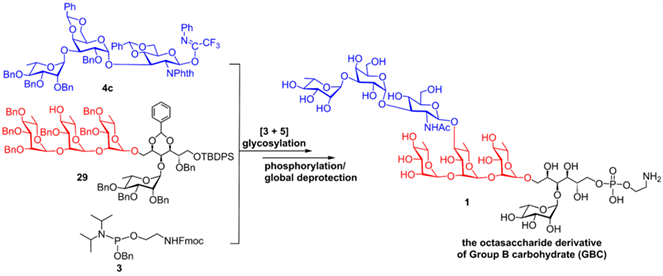
Group B Streptococcus (GBS) is the major pathogen that causes invasive infectious diseases in neonates and infants. The development of preventive and therapeutic strategies against GBS infection has been becoming the most pressing subject worldwide. Group B carbohydrate (GBC), the group B-specific polysaccharide that distinguishes GBS with other streptococci species, has been identified as an attractive antigen for diagnosis and vaccine development because of its highly conservative tetra-antennary structure. In this paper, a highly convergent [3 + 5] glycosylation strategy for efficient synthesis of an octasaccharide derivative related to GBC oligosaccharide unit II has been developed. In this synthesis, each glycosylation reaction was efficiently constructed with glycosyl imidates, especially trifluoroacetimidate, as donors, and each glycosidic bond was stereoselectively controlled via the neighboring group participation effect of acyl group on the 2-O-position of imidate donors or the solvent effect of Et2O. Furthermore, the aminoethylphosphate group was smoothly installed on the 6-O-position of d-glucitol residue using the phosphoramidite method. After global deprotection, the target octasaccharide was successfully obtained from d-glucitol in 29 steps with an overall yield of 1.37%. The free amino group installed on the aminoethylphosphate spacer of the target molecule enables its modification with functionalized biomolecules for further biological studies.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200544-sup-0001-Supinfo.pdfPDF document, 8.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Madrid, L.; Seale, A. C.; Kohli-Lynch, M.; Edmond, K. M.; Lawn, J. E.; Heath, P. T.; Madhi, S. A.; Baker, C. J.; Bartlett, L.; Cutland, C.; Gravett, M. G.; Ip, M.; Le Doare, K.; Rubens, C. E.; Saha, S. K.; Sobanjo-Ter Meulen, A.; Vekemans, J.; Schrag, S. Infant GBS Disease Investigator Group. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S160−S172.
- 2 Le Doare, K.; Heath, P. T. An Overview of Global GBS Epidemiology. Vaccine 2013, 31, D7–D12.
- 3 Seale, A. C.; Bianchi-Jassir, F.; Russell, N. J.; Kohli-Lynch, M.; Tann, C. J.; Hall, J.; Madrid, L.; Blencowe, H.; Cousens, S.; Baker, C. J.; Bartlett, L.; Cutland, C.; Gravett, M. G.; Heath, P. T.; Ip, M.; Le Doare, K.; Madhi, S. A.; Rubens, C. E.; Saha, S. K.; Schrag, S. J.; Sobanjo-ter Meulen, A.; Vekemans, J.; Lawn, J. E. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017, 65, S200–S219.
- 4 Libster, R.; Edwards, K. M.; Levent, F.; Edwards, M. S.; Rench, M. A.; Castagnini, L. A.; Cooper, T.; Sparks, R. C.; Baker, C. J.; Shah, P. E. Long-term Outcomes of Group B Streptococcal Meningitis. Pediatrics 2012, 130, e8–e15.
- 5 Raabe, V. N.; Shane, A. L. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr. 2019, 7, 228–238.
- 6 Patras, K. A.; Nizet, V. Group B Streptococcal Maternal Colonization and Neonatal Disease: Molecular Mechanisms and Preventative Approaches. Front. Pediatr. 2018, 6, 27.
- 7 Baker, C. J. The Spectrum of Perinatal Group B Streptococcal Disease. Vaccine 2013, 31, D3–D6.
- 8Seale, A. C.; Koech, A. C.; Sheppard, A. E.; Barsosio, H. C.; Langat, J.; Anyango, E.; Mwakio, S.; Mwarumba, S.; Morpeth, S. C.; Anampiu, K.; Vaughan, A.; Giess, A.; Mogeni, P.; Walusuna, L.; Mwangudzah, H.; Mwanzui, D.; Salim, M.; Kemp, B.; Jones, C.; Mturi, N.; Tsofa, B.; Mumbo, E.; Mulewa, D.; Bandika, V.; Soita, M.; Owiti, M.; Onzere, N.; Walker, A. S.; Schrag, S. J.; Kennedy, S. H.; Fegan, G.; Crook, D. W.; Berkley, J. A. Maternal Colonization with Streptococcus agalactiae and Associated Stillbirth and Neonatal Disease in Coastal Kenya. Nat. Microbiol. 2016, 1, 16067–16110.
- 9 Kobayashi, M.; Schrag, S. J.; Alderson, M. R.; Madhi, S. A.; Baker, C. J.; Sobanjoter Meulen, A.; Kaslow, D. C.; Smith, P. G.; Moorthy, V. S.; Vekemans, J. WHO Consultation on Group B Streptococcus Vaccine Development: Report from a Meeting Held on 27–28 April 2016. Vaccine 2019, 37, 7307−7314.
- 10 Davies, S.; Gear, J. E.; Mason, C. M.; Mcintyre, S. M.; Hall, L. Streptococcus grouping latex kits: evaluation of five commercially available examples. Br. J. Biomed. Sci. 2003, 60, 136–140.
- 11 Seeberger, P. H. Discovery of Semi- and Fully-synthetic Carbohydrate Vaccines against Bacterial Infections Using a Medicinal Chemistry Approach. Chem. Rev. 2021, 121, 3598–3626.
- 12 Del Bino, L.; Østerlid, K. E.; Wu, D.; Nonne, F.; Romano, M. R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, doi: https://doi.org/10.1021/acs.chemrev.2c00021.
- 13 Lancefield, R. C. A Serological Differentiation of Specific Types of Bovine Hemolytic Streptococci (Group B). J. Exp. Med. 1934, 59, 441−458.
- 14 Deng, L.; Kasper, D. L.; Krick, T. P.; Wessels, M. R. Characterization of the Linkage between the Type III Capsular Polysaccharide and the Bacterial Cell Wall of Group B Streptococcus. J. Biol. Chem. 2000, 275, 7497−7504.
- 15 Cieslewicz, M. J.; Chaffin, D.; Glusman, G.; Kasper, D.; Madan, A.; Rodrigues, S.; Fahey, J.; Wessels, M. R.; Rubens, C. E. Structural and Genetic Diversity of Group B Streptococcus Capsular Polysaccharides. Infect. Immun. 2005, 73, 3096−3103.
- 16 Nuccitelli, A.; Rinaudo, C. D.; Maione, D. Group B Streptococcus Vaccine: State of the Art. Ther. Adv. Vaccines 2015, 3, 76–90.
- 17 Carreras-Abad, C.; Ramkhelawon, L.; Heath, P. T.; Le Doare, K. A Vaccine against Group B Streptococcus: Recent Advances. Infect. Drug Resist. 2020, 13, 1263–1272.
- 18 Liao, G.; Guo, J.; Yang, D.; Zhou, Z.; Liu, Z.; Guo, Z. Synthesis of a Dimer of the Repeating Unit of Type Ia Group B Streptococcus Extracellular Capsular Polysaccharide and Immunological Evaluations of Related Protein Conjugates. Org. Chem. Front. 2019, 6, 2833–2838.
- 19 Absalon, J.; Segall, N.; Block, S. L.; Center, K. J.; Scully, I. L.; Giardina, P. C.; Peterson, J.; Watson, W. J.; Gruber, W. C.; Jansen, K. U.; Peng, Y.; Munson, S.; Pavliakova, D.; Scott, D. A.; Anderson, A. S. Safety and Immunogenicity of a Novel Hexavalent Group B Streptococcus Conjugate Vaccine in Healthy, Non-pregnant Adults: A Phase 1/2, Randomised, Placebo-controlled, Observer-blinded, Dose-escalation Trial. Lancet Infect. Dis. 2021, 21, 263−274
- 20 Madhi, S. A.; Cutland, C. L.; Jose, L.; Koen, A.; Govender, N.; Wittke, F.; Olugbosi, M.; Meulen, A. S.; Baker, S.; Dull, P. M.; Narasimhan, V.; Slobod, K. Safety and Immunogenicity of an Investigational Maternal Trivalent Group B Streptococcus Vaccine in Healthy Women and Their Infants: A Randomised Phase 1b/2 Trial. Lancet Infect. Dis. 2016, 16, 923−934.
- 21 Greenberg, D. N.; Ascher, D. P.; Yoder, B. A.; Hensley, D. M.; Heiman, H. S.; Keith, J. F. Sensitivity and Specificity of Rapid Diagnostic Tests for Detection of Group B Streptococcal Antigen in Bacteremic Neonates. J. Clin. Microbiol. 1995, 33, 193−198.
- 22 Anthony, B. F.; Concepcion, N. F.; Concepcion, K. F. Human Antibody to the Group-Specific Polysaccharide of Group B Streptococcus. J. Infect. Dis. 1985, 151, 221-226.
- 23 Marques, M. B.; Kasper, D. L.; Shroff, A.; Michon, F.; Jennings, H. J.; Wessels, M. R. Functional Activity of Antibodies to the Group B Polysaccharide of Group B Streptococci Elicited by a Polysaccharide-Protein Conjugate Vaccine. Infect. Immun. 1994, 62, 1593−1599.
- 24 Wang, Z.; Enotarpi, J.; Buffi, G.; Pezzicoli, A.; Gstöttner, C. J.; Nicolardi, S.; Balducci, E.; Fabbrini, M.; Romano, M. R.; van der Marel, G. A.; Del Bino, L.; Adamo, R.; Codée, J. D. C. Chemical Synthesis and Immunological Evaluation of Fragments of the Multiantennary Group-Specific Polysaccharide of Group B Streptococcus. JACS Au 2022, 2, 1724−1735.
- 25 Michon, F.; Katzenellenbogen, E.; Kasper, D. L.; Jennings, H. J. Structure of the Complex Group-Specific Polysaccharide of Group B Streptococcus. Biochemistry 1987, 26, 476−486.
- 26 Michon, F.; Brisson, J. R.; Dell, A.; Kasper, D. L.; Jennings, H. J. Multiantennary Group-specific Polysaccharide of Group B Streptococcus. Biochemistry 1988, 27, 5341−5351.
- 27 Pozsgay, V.; Brisson, J. R.; Jennings, H. J. Synthetic Oligosaccharides Related to Group B Streptococcal Polysaccharides. The Rhamnotriose Moiety of the Common Antigen. Can. J. Chem. 1987, 65, 2764−2769.
- 28 Pozsgay, V.; Jennings, H. J. Synthesis of a Di-, Tri-, and Tetrasaccharide Unit of the Group B Streptococcal Common Antigen. Carbohydr. Res. 1988, 179, 61−75.
- 29 Pozsgay, V.; Jennings, H. J. Synthesis of Oligosaccharides Corresponding to the Common Polysaccharide Antigen of Group B Streptococci. J. Org. Chem. 1988, 53, 4042−4052.
- 30 Michon, F.; Chalifour, R.; Feldman, R.; Wessels, M.; Kasper, D. L.; Gamian, A.; Pozsgay, V.; Jennings, H. J. The α-l-(1→2)-Trirhamnopyranoside Epitope on the Group-Specific Polysaccharide of Group B Streptococci. Infect. Immun. 1991, 59, 1690−1696.
- 31 Wu, Y.; Xiong, D.; Chen, S.; Wang, Y.; Ye, X. Total synthesis of Mycobacterial Arabinogalactan Containing 92 Monosaccharide Units. Nat. Commun. 2017, 8, 14851.
- 32 Joseph, A. A.; Pardo-Vargas, A.; Seeberger, P. H. Total Synthesis of Polysaccharides by Automated Glycan Assembly. J. Am. Chem. Soc. 2020, 142, 8561−8564.
- 33 Zhu, Q.; Shen, Z.; Chiodo, F.; Nicolardi, S.; Molinaro, A.; Silipo, A.; Yu, B. Chemical Synthesis of Glycans Up to a 128-mer Relevant to the O-antigen of Bacteroides vulgatus. Nat. Commun. 2020, 11, 4142.
- 34 Lei, J.-C.; Jiang, Y.-Y.; Xia, Y.-F.; Fang, Q.; Duan, S.-C.; Ruan, Y.-X.; Yang, J.-S. Stereoselective Synthesis of a Tetrasaccharide Fragment from Rhamnogalacturonan II Side Chain A. Chin. J. Chem. 2022, 40, 1799−1806.
- 35 Qin, X.; Ye, X.-S. Donor Preactivation-Based Glycosylation: An Efficient Strategy for Glycan Synthesis. Chin. J. Chem. 2021, 39, 531−542.
- 36 Li, X.-Q; Ma, Z.; Liu, R.-K; Hurevich, M.; Yang, Y. Photolabile Protecting Group-Mediated Synthesis of 2-Deoxy-Glycosides. Chin. J. Chem. 2021, 39, 3309−3314.
- 37 Liu, X.; Lin, Y.; Liu, A.; Sun, Q.; Sun, H.; Xu, P.; Li, G.; Song, Y.; Xie, W.; Sun, H.; Yu, B.; Li, W. 2-Diphenylphosphinoyl-acetyl as a Remote Directing Group for the Highly Stereoselective Synthesis of β-Glycosides. Chin. J. Chem. 2022, 40, 443−452.
- 38 Li, X.; Yang, Y. Automated Chemical Solid-Phase Synthesis of Glycans. Chin. J. Chem. 2022, 40, 1714−1728.
- 39 Liang, M.; Gong, W.; Sun, C.; Zhao, J.; Wang, H.; Chen, Z.; Xiao, M.; Gu, G. Sequential One-Pot Three-Enzyme Synthesis of the Tetrasaccharide Repeating Unit of Group B Streptococcus Serotype VIII Capsular Polysaccharide. Chin. J. Chem. 2022, 40, 1039−1044.
- 40 Xue, J.; Guo, Z. Convergent Synthesis of a GPI Containing an Acylated Inositol. J. Am. Chem. Soc. 2003, 125, 16334−16339.
- 41 Reddy, M. S.; Zhang, H.; Phoenix, S.; Deslongchamps, P. Total Synthesis of Ouabagenin and Ouabain. Chem. Asian J. 2009, 4, 725−741.
- 42 Bartetzko, M. P.; Schuhmacher, F.; Hahm, H. S.; Seeberger, P. H.; Pfrengle, F. Automated Glycan Assembly of Oligosaccharides Related to Arabinogalactan Proteins. Org. Lett. 2015, 17, 4344−4347.
- 43 Zhang, Z.; Ollmann, I. R.; Ye, X.; Wischnat, R.; Baasov, T.; Wong, C. Programmable One-Pot Oligosaccharide Synthesis. J. Am. Chem. Soc. 1999, 121, 734–753.
- 44 Yu, B.; Tao, H.-C. Glycosyl Trifluoroacetimidates. Part 1: Preparation and Application as New Glycosyl Donors. Tetrahedron Lett. 2001, 42, 2405–2407.
- 45 Yu, C.; Wang, H.; Chiang, L.; Pei, K. Synthesis of the Rhamnosyl Trisaccharide Repeating Unit to Mimic the Antigen Determinant of Pseudomonas syringae Lipopolysaccharide. Synthesis 2007, 1412−1420.
- 46 Scanlan, E. M.; Mackeen, M. M.; Wormald, M. R.; Davis, B. G. Synthesis and Solution-Phase Conformation of the RG-I Fragment of the Plant Polysaccharide Pectin Reveals a Modification-modulated Assembly Mechanism. J. Am. Chem. Soc. 2010, 132, 7238−7239.
- 47 Gu, G.; Du, Y.; Linhardt, R. J. Facile Synthesis of Saponins Containing 2,3-Branched Oligosaccharides by Using Partially Protected Glycosyl Donors. J. Org. Chem. 2004, 69, 5497−5500.
- 48 Xu, F. F.; Pereira, C. L.; Seeberger, P. H. 1,3-Dibromo-5,5-dimethylhydantoin as Promoter for Glycosylations Using Thioglycosides. Beilstein J. Org. Chem. 2017, 13, 1994−1998.
- 49 Kafle, A.; Liu, J.; Cui, L. Controlling the Stereoselectivity of Glycosylation via Solvent Effects. Can. J. Chem. 2016, 94, 894−901.
- 50 Tvaroška, I.; Bleha, T. Anomeric and Exo-anomeric Effects in Carbohydrate Chemistry. Adv. Carbohydr. Chem. Biochem. 1989, 47, 45−123.
- 51 Kuszmann, J.; Medgyes, G.; Boros, S. Two Approaches to the Synthesis of 3-β-d-glucopyranosyl-d-glucitol. Carbohydr. Res. 2004, 339, 2407−2414.
- 52 Brecknell, D. J.; Carman, R. M.; Kibby, J. J.; Nicholas, L. T. Tri-O-Benzylidene-d-glucitol. Aust. J. Chem. 1976, 29, 1859−1863.
- 53 Bock, K.; Pedersen, C. A study of 13CH Coupling Constants in Hexopyranoses. J. Chem. Soc., Perkin Trans. 2 1974, 293−297.
- 54
Nukada, T.; Lucas, H.; Konradsson, P.; van Boeckel, C. A. A. Syntheses of Larger Modified Oligosaccharides Containing “Opened Carbohydrate” Fragments. Synlett 1991, 5, 365–368.
10.1055/s-1991-20732 Google Scholar
- 55 Braga, R. M.; Kato, L. A Formal Total Synthesis of Deoxynojirimycin from d-glucitol. J. Braz. Chem. Soc. 2003, 14, 822−827.
- 56 Milhomme, O.; John, C.; Djedaïni-Pilard, F.; Grandjean, C. Access to Antigens Related to Anthrose Using Pivotal Cyclic Sulfite/Sulfate Intermediates. J. Org. Chem. 2011, 76, 5985−5998.
- 57 Okada, Y.; Ohtsu, M.; Bando, M.; Yamada, H. Benzyl N-Phenyl- 2,2,2-trifluoroacetimidate: A New and Stable Reagent for O-Benzylation. Chem. Lett. 2007, 36, 992–993.
- 58 Shekhani, M. S.; Khan, K. M.; Mahmood, K.; Shah, P. M.; Malik, S. Selective Cleavage of t-Butyldiphenylsilyl Ethers in the Presence of t-Butyldimethylsilyl Ethers. Tetrahedron Lett. 1990, 31, 1669−1670.




