Palladium-Catalyzed Tandem Cyclization Strategy for the Assembly of 3-Halo-1,2,5-triarylpyrroles from N-Alkylanilines and Haloalkynes
Songjia Fang
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorHuanfeng Jiang
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Wanqing Wu
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]Search for more papers by this authorSongjia Fang
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorHuanfeng Jiang
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Wanqing Wu
Key Laboratory of Functional Molecular Engineering of Guangdong Province, State Key Laboratory of Luminescent Materials and Devices, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
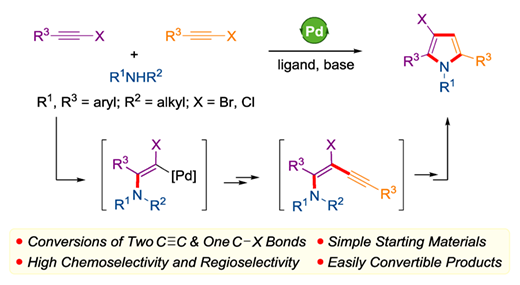
This report discloses a distinctive palladium-catalyzed sequential tandem cyclization reaction of two molecular haloalkynes and one molecular N-alkylanilines, leading to the efficient assembly of various 3-halo-1,2,5-triarylpyrrole derivatives. Two carbon-carbon triple bonds and one carbon-halogen bond in two molecular haloalkynes are transformed conveniently in one single step, which may involve the aminoalkynylation of haloalkyne and cyclization of the newly formed enyne intermediate. The high chemo- and regioselectivities, good functional group tolerance and late-stage modification of the halopyrrole products further illustrate the synthetic value of this strategy.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200422-sup-0001-Supinfo.pdfPDF document, 5.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Wu, W.; Jiang, H. Haloalkynes: A Powerful and Versatile Building Block in Organic Synthesis. Acc. Chem. Res. 2014, 47, 2483–2504;
(b) Jiang, H.; Zhu, C.; Wu, W. Haloalkyne Chemistry, Springer, Heidelberg, 2016, pp. 1—77;
10.1007/978-3-662-49001-3_1 Google Scholar(c) Petko, D.; Koh, S.; Tam, W. Transition Metal- Catalyzed Reactions of Alkynyl Halides. Curr. Org. Synth. 2019, 16, 546–582; (d) Zhao, Y.; Jin, J.; Chan, P. W. H. Gold Catalyzed Photoredox C1-Alkynylation of N-Alkyl-1,2,3,4-tetrahydroisoquinolines by 1-Bromoalkynes with UVA Led Light. Adv. Synth. Catal. 2019, 361, 1313–1321; (e) Kreuzahler, M.; Haberhauer, G. Metal-Catalyzed Haloalkynylation Reactions. Chem. - Eur. J. 2022, 28, e202103046.
- 2(a) Chen, D.; Cao, Y.; Yuan, Z.; Cai, H.; Zheng, R.; Kong, L.; Zhu, G. Synthesis of cis-1,2-Dihaloalkenes Featuring Palladium-Catalyzed Coupling of Haloalkynes and α,β-Unsaturated Carbonyls. J. Org. Chem. 2011, 76, 4071–4074; (b) Li, J.; Yang, W.; Yang, S.; Huang, L.; Wu, W.; Sun, Y.; Jiang, H. Palladium-Catalyzed Cascade Annulation to Construct Functionalized β- and γ-Lactones in Ionic Liquids. Angew. Chem. Int. Ed. 2014, 53, 7219–7222; (c) Li, J.; Yang, S.; Jiang, H.; Wu, W.; Zhao, J. Palladium-Catalyzed Coupling of Alkynes with Unactivated Alkenes in Ionic Liquids: A Regio- and Stereoselective Synthesis of Functionalized 1,6-Dienes and Their Analogues. J. Org. Chem. 2013, 78, 12477–12486; (d) Zheng, J.; Huang, L.; Li, Z.; Wu, W.; Li, J.; Jiang, H. Synthesis of 3-Bromosubstituted Pyrroles via Palladium-Catalyzed Intermolecular Oxidative Cyclization of Bromoalkynes with N-Allylamines. Chem. Commun. 2015, 51, 5894–5897; (e) Ji, X.; Nie, J.; Peng, X.; Hu, J.; Xu, X.; Huang, Y.; Li, Y.; Jiang, H. Palladium-Catalyzed Cross Haloalkynylation of Haloalkynes. Org. Lett. 2022, 24, 3384–3388.
- 3(a) Nicolai, S.; Sedigh-Zadeh, R.; Waser, J. Pd(0)-Catalyzed Alkene Oxy- and Aminoalkynylation with Aliphatic Bromoacetylenes. J. Org. Chem. 2013, 78, 3783–3801; (b) Suseelan Sarala, A.; Bhowmick, S.; Carvalho, R. L.; Al-Thabaiti, S. A.; Mokhtar, M.; Silva Júnior, E. N.; Maiti, D. Transition-Metal-Catalyzed Selective Alkynylation of C-H Bonds. Adv. Synth. Catal. 2021, 363, 4994–5027; (c) Li, M.; Fang, S.; Zheng, J.; Jiang, H.; Wu, W. Direct Assembly of Polysubstituted Propiolamidinates via Palladium-Catalyzed Multicomponent Reaction of Isocyanides. Org. Lett. 2019, 21, 8439–8443; (d) Liu, B.; Ouyang, W.; Nie, J.; Gao, Y.; Feng, K.; Huo, Y.; Chen, Q.; Li, X. Weak Coordinated Nitrogen Functionality Enabled Regioselective C-H Alkynylation via Pd(II)/Mono-N-Protected Amino Acid Catalysis. Chem. Commun. 2020, 56, 11255–11258; (e) Fang, S.; Jiang, G.; Li, M.; Liu, Z.; Jiang, H.; Wu, W. Palladium-Catalyzed Regioselective C-H Alkynylation of Indoles with Haloalkynes: Access to Functionalized 7-Alkynylindoles. Chem. Commun. 2019, 55, 13769–13772; (f) Ano, Y.; Kawai, N.; Chatani, N. Palladium-Catalyzed 1,1-Alkynylbromination of Alkenes with Alkynyl Bromides. Chem. Sci. 2021, 12, 12326–12332; (g) Guo, L.-Y.; Li, Q.; Liu, Y.-T.; Li, L.; Ni, Y.-Q.; Li, Y.; Pan, F. Palladium- Catalyzed Alkynylation of Alkenes via C-H Activation for the Preparation of Conjugated 1,3-Enynes. Adv. Synth. Catal. 2022, 364, 1109–1116; (h) Porey, S.; Zhang, X.; Bhowmick, S.; Kumar Singh, V.; Guin, S.; Paton, R. S.; Maiti, D. Alkyne Linchpin Strategy for Drug: Pharmacophore Conjugation: Experimental and Computational Realization of a Meta-Selective Inverse Sonogashira Coupling. J. Am. Chem. Soc. 2020, 142, 3762–3774; (i) Yang, C.; Li, F.; Wu, T.-R.; Cui, R.; Wu, B.-B.; Jin, R.-X.; Li, Y.; Wang, X.-S. Development of Axially Chiral Styrene-Type Carboxylic Acid Ligands via Palladium-Catalyzed Asymmetric C-H Alkynylation. Org. Lett. 2021, 23, 8132–8137.
- 4(a) Fan, H.; Peng, J.; Hamann, M. T.; Hu, J. F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287; (b) Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274; (c) Estevez, V.; Villacampa, M.; Menéndez, J. C. Recent Advances in the Synthesis of Pyrroles by Multicomponent Reactions. Chem. Soc. Rev. 2014, 43, 4633–4657; (d) Bianco, M.; Marinho, D.; Hoelz, L. V. B.; Bastos, M. M.; Boechat, N. Pyrroles as Privileged Scaffolds in the Search for New Potential HIV Inhibitors. Pharmaceuticals 2021, 14, 893–910; (e) Singh, N.; Singh, S.; Kohli, S.; Singh, A.; Asiki, H.; Rathee, G.; Chandra, R.; Anderson, E. A. Recent Progress in the Total Synthesis of Pyrrole-Containing Natural Products (2011–2020). Org. Chem. Front. 2021, 8, 5550–5573; (f) Xiong, J.; Mu, Z.-Y.; Yao, G.; Zhang, J.-A.; Feng, Q.-X.; He, H.-T.; Pang, Y.-L.; Shi, H.; Ding, M.-W. One-Pot Synthesis of Polysubstituted Pyrroles via SequentialKetenimine Formation/Ag(I)-Catalyzed Alkyne Cycloisomerisation Starting from Ylide Adducts. Chin. J. Chem. 2021, 39, 1553–1557; (g) Arafa, W. A. A.; Hussein, M. F. Design, Sonosynthesis, Quantum-Chemical Calculations, and Evaluation of New Mono- and Bis-Pyridine Dicarbonitriles as Antiproliferative Agents. Chin. J. Chem. 2020, 38, 501–508.
- 5 Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. A Submarine Journey: The Pyrrole-Imidazole Alkaloids. Mar. Drugs 2009, 7, 705–753; (b) Clive, D. L. J.; Cheng, P. The Marinopyrroles. Tetrahedron 2013, 69, 5067–5078; (c) Pina, I. C.; White, K. N.; Cabrera, G.; Rivero, E.; Crews, P. J. Nat. Prod. 2007, 70, 613–617; (d) Kovalerchik, D.; Singh, R. P.; Schlesinger, P.; Mahajni, A.; Shefer, S.; Fridman, M.; Ilan, M.; Carmeli, S. Bromopyrrole Alkaloids of the Sponge Agelas Oroides Collected near the Israeli Mediterranean Coastline. J. Nat. Prod. 2020, 83, 374–384.
- 6(a) Hewlett, N. M.; Tepe, J. J. Total Synthesis of the Natural Product (±)-Dibromophakellin and Analogues. Org. Lett. 2011, 13, 4550–4553; (b) Sakaguchi, H.; Uetake, Y.; Ohashi, M.; Niwa, T.; Ogoshi, S.; Hosoya, T. Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes En Route to Diverse Fluoroalkenes. J. Am. Chem. Soc. 2017, 139, 12855–12862; (c) Inaba, K.; Iwai, R.; Morimoto, M.; Irie, M. Thermally Reversible Photochromism of Dipyrrolylethenes. Photochem. Photobiol. Sci. 2019, 18, 2136–2141; (d) Yamaguchi, A. D.; Chepiga, K. M.; Yamaguchi, J.; Itami, K.; Davies, H. M. Concise Syntheses of Dictyodendrins A and F by a Sequential C-H Functionalization Strategy. J. Am. Chem. Soc. 2015, 137, 644–647; (e) Morii, K.; Yasuda, Y.; Morikawa, D.; Mori, A.; Okano, K. Total Synthesis of Lamellarins G, J, L, and Z Using One-Pot Halogen Dance/ Negishi Coupling. J. Org. Chem. 2021, 86, 13388–13401.
- 7(a) Gilow, H. M.; Burton, D. E. Bromination and Chlorination of Pyrrole and Some Reactive 1-Substituted Pyrroles. J. Org. Chem. 1981, 46, 2221–2225; (b) Kozikowski, A. P.; Cheng, X.-M. A Synthesis of 3-Substituted Pyrroles through the Halogen-Metal Exchange Reaction of 3-Bromo-1-(triisopropylsilyl) Pyrrole. J. Org. Chem. 1984, 49, 3239–3240; (c) Huang, C.; Zeng, Y.; Cheng, H.; Hu, A.; Liu, L.; Xiao, Y.; Zhang, J. A One-Pot Construction of Halogenated Trifluoromethylated Pyrroles through NXS (X = Br, I) Triggered Cascade. Org. Lett. 2017, 19, 4968–4971; (d) Debrouwer, W.; Heugebaert, T. S. A.; Stevens, C. V. Preparation of Tetrasubstituted 3-Phosphonopyrroles through Hydroamination: Scope and Limitations. J. Org. Chem. 2014, 79, 4322–4331.
- 8(a) De Kimpe, N.; Tehrani, K. A.; Stevens, C.; De Cooman, P. Synthesis of 3-Halopyrroles. Tetrahedron 1997, 53, 3693–3706; (b) Merkul, E.; Boersch, C.; Frank, W.; Müller, T. J. J. Three-Component Synthesis of N-Boc-4-iodopyrroles and Sequential One-Pot Alkynylation. Org. Lett. 2009, 11, 2269–2272; (c) Sai, M.; Matsubara, S. Silver-Catalyzed Intramolecular Chloroamination of Allenes: Easy Access to Functionalized 3-Pyrroline and Pyrrole Derivatives. Org. Lett. 2013, 13, 4676–4679; (d) Bharathiraja, G.; Sakthivel, S.; Sengoden, M.; Punniyamurthy, T. A Novel Tandem Sequence to Pyrrole Syntheses by 5-endo-dig Cyclization of 1,3-Enynes with Amines. Org. Lett. 2013, 13, 4996–4999.
- 9 Gao, Y.; Yin, M.; Wu, W.; Huang, H.; Jiang, H. Copper-Catalyzed Intermolecular Oxidative Cyclization of Haloalkynes: Synthesis of 2-Halo-substituted Imidazo[1,2-a]pyridines, Imidazo[1,2-a]pyrazines and Imidazo[1,2-a]pyrimidines. Adv. Synth. Catal. 2013, 355, 2263–2273.
- 10(a) Wang, S.; Li, P.; Yu, L.; Wang, L. Sequential and One-Pot Reactions of Phenols with Bromoalkynes for the Synthesis of (Z)-2-Bromovinyl Phenyl Ethers and Benzo[b]furans. Org. Lett. 2011, 13, 5968–5971; (b) Jiang, G.; Li, J.; Zhu, C.; Wu, W.; Jiang, H. Palladium-Catalyzed Sequential Nucleophilic Addition/Oxidative Annulation of Bromoalkynes with Benzoic Acids to Construct Functionalized Isocoumarins. Org. Lett. 2017, 19, 4440–4443; (c) Pigulski, B.; Męcik, P.; Cichos, J.; Szafert, S. Use of Stable Amine-Capped Polyynes in the Regioselective Synthesis of Push-Pull Thiophenes. J. Org. Chem. 2017, 82, 1487–1498; (d) Mishiro, K.; Yushima, Y.; Kunishima, M. Phototriggered Dehydration Condensation Using an Aminocyclopropenone. Org. Lett. 2017, 19, 4912–4915.
- 11(a) Huang, L.; Olivares, A. M.; Weix, D. J. Reductive Decarboxylative Alkynylation of N-Hydroxyphthalimide Esters with Bromoalkynes. Angew. Chem. Int. Ed. 2017, 56, 11901–11905; (b) Jiang, G.; Zhu, C.; Li, J.; Wu, W.; Jiang, H. Silver-Catalyzed Regio- and Stereoselective Thiocyanation of Haloalkynes: Access to (Z)-Vinyl Thiocyanates. Adv. Synth. Catal. 2017, 359, 1208–1212.
- 12 Brotzel, F.; Chu, Y. C.; Mayr, H. Nucleophilicities of Primary and Secondary Amines in Water. J. Org. Chem. 2007, 72, 3679–3688.
- 13(a) Feng, X.; Tong, B.; Shen, J.; Shi, J.; Han, T.; Chen, L.; Zhi, J.; Lu, P.; Ma, Y.; Dong, Y. Aggregation-Induced Emission Enhancement of Aryl-Substituted Pyrrole Derivatives. J. Phys. Chem. B 2010, 114, 16731–16736; (b) Lyons, T. W.; Martinot, T. A.; He, C. Q.; Qi, J.; Shao, G. Development of a Zinc-Mediated Approach to a 2,3-cis-Pyrrolidine Arginase Inhibitor. Org. Process Res. Dev. 2020, 24, 1457–1466; (c) Morán-Poladura, P.; Rubio, E.; González, J. M. Intramolecular C-H Activation through Gold(I)-Catalyzed Reaction of Iodoalkynes. Angew. Chem. Int. Ed. 2015, 54, 3052–3055; (d) Mital, A.; Murugesan, D.; Kaiser, M.; Yeates, C.; Gilbert, I. H. Discovery and Optimisation Studies of Antimalarial Phenotypic Hits. Eur. J. Med. Chem. 2015, 103, 530–538.
- 14(a) Li, Y.; Liu, X.; Jiang, H.; Feng, Z. Expedient Synthesis of Functionalized Conjugated Enynes: Palladium-Catalyzed Bromoalkynylation of Alkynes. Angew. Chem. Int. Ed. 2010, 49, 3338–3341; (b) Kalyani, D.; Deprez, N. R.; Desai, L. V.; Sanford, M. S. Oxidative C-H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J. Am. Chem. Soc. 2005, 127, 7330–7331; (c) Ye, X.; Xu, C.; Wojtas, L.; Akhmedov, N. G.; Chen, H.; Shi, X. Silver-Free Palladium- Catalyzed sp3 and sp2 C-H Alkynylation Promoted by a 1,2,3-Triazole Amine Directing Group. Org. Lett. 2016, 18, 2970–2973.
- 15(a) Debnath, S.; Lu, M.; Liang, L.; Shi, Y. A Tandem Nucleophilic Aminopalladation and Carbene Insertion Sequence for Indole Fused Polycycles. Org. Lett. 2021, 23, 7118–7122; (b) Luo, Y.-G.; Basha, R.-S.; Reddy, D. M.; Xue, Y.-J.; Chen, T.-H.; Lee, C.-F. Palladium-Catalyzed Synthesis of 2,3-Diaryl-N-Methylindoles from ortho-Alkynylanilines and Aryl Pinacol Boronic Esters. Org. Lett. 2018, 20, 6872–6876; (c) Li, J.; Lin, Z.; He, D.; Wu, W.; Jiang, H. Palladium-Catalyzed Sequential Cyclization/Functionalization of Oxime Ethers with Unactivated Vinyl Ethers for Tunable Assembly of Structurally Diverse Isoxazoles. Chin. J. Chem. 2021, 39, 3285–3291; (d) Jin, T.; Suzuki, S.; Ho, H. E.; Matsuyama, H.; Kawata, M.; Terada, M. Pd-Catalyzed Indolization/ peri-C-H Annulation/N-Dealkylation Cascade to Cyclopenta-Fused Acenaphtho[1,2-b]Indole Scaffold. Org. Lett. 2021, 23, 9431–9435.




