Stable Boron-Containing Blue-Photoluminescent Radicals
Zhongtao Feng
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
‡These authors contributed equally to this work.
Search for more papers by this authorYuanyuan Chong
Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui, 230026 China
‡These authors contributed equally to this work.
Search for more papers by this authorShuxuan Tang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorHuapeng Ruan
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorYong Fang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorYue Zhao
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorCorresponding Author
Jun Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Xinping Wang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
E-mail: [email protected], [email protected]Search for more papers by this authorZhongtao Feng
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
‡These authors contributed equally to this work.
Search for more papers by this authorYuanyuan Chong
Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui, 230026 China
‡These authors contributed equally to this work.
Search for more papers by this authorShuxuan Tang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorHuapeng Ruan
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorYong Fang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorYue Zhao
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
Search for more papers by this authorCorresponding Author
Jun Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Xinping Wang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210093 China
E-mail: [email protected], [email protected]Search for more papers by this authorMain observation and conclusion
It is highly urgent to develop synthetic strategies to make new category of stable luminescent radicals with desired emission wavelength. In this study, we have isolated two dioxoborocyclic radicals (3 and 4) by a direct synthetic route. They were characterized by UV, EPR spectroscopy and SQUID measurements. Their structures were obtained by single-crystal X-ray diffraction. Both radicals produce blue-photoluminescence (458 nm for 3 and 478 nm for 4) by radiative decay from higher excited states (D2/D3) to the ground state (D0) based on theoretical calculation, breaking Kasha rule. The work records a new kind of radical emitters and the first stable radicals with blue emission bands.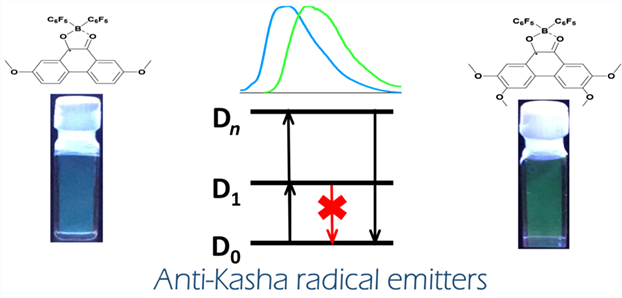
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100188-sup-0001-Supinfo.pdfPDF document, 935.8 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Tang, C. W.; VanSlyke, S. A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915; (b) Zhu, X. H.; Peng, J.; Cao, Y.; Roncali, J. Solution-Processable Single-Material Molecular Emitters for Organic Light-Emitting Devices. Chem. Soc. Rev. 2011, 40, 3509–3524; (c) Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent Progress in Metal-Organic Complexes for Optoelectronic Applications. Chem. Soc. Rev. 2014, 43, 3259–3302; (d) Xu, R.; Li, Y.; Tang, J. Recent Advances in Flexible Organic Light-Emitting Diodes. J. Mater. Chem. C 2016, 4, 9116–9142.
- 2(a) Cui, Z.; Abdurahman, A.; Ai, X.; Li, F. Stable Luminescent Radicals and Radical-Based LEDs with Doublet Emission. CCS Chem. 2020, 2, 1129–1145;
(b) Ai, X.; Evans, E. W.; Dong, S.; Gillett, A. J.; Guo, H.; Chen, Y.; Hele, T. J. H.; Friend, R. H.; Li, F. Efficient Radical-Based Light-Emitting Diodes with Doublet Emission. Nature 2018, 563, 536–540;
(c) Abdurahman, A.; Hele, T. J. H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R. H.; Li, F.; Evans, E. W. Understanding the Luminescent Nature of Organic Radicals for Efficient Doublet Emitters and Pure-Red Light-Emitting Diodes. Nat. Mater. 2020, 19, 1224–1229;
(d) Peng, Q.; Obolda, A.; Zhang M.; Li, F. Organic Light-Emitting Diodes Using a Neutral π-Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 127, 7197–7201;
10.1002/ange.201500242 Google Scholar(e) Hattori, Y.; Kusamoto, T.; Nishihara, H. Luminescence, Stability, and Proton Response of an Open-Shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl Radical. Angew. Chem. Int. Ed. 2014, 44, 11845–11848; (f) Hattori, Y.; Kusamoto, T.; Nishihara, H. Enhanced Luminescent Properties of an Open-Shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl Radical by Coordination to Gold. Angew. Chem. Int. Ed. 2015, 54, 3731–3734; (g) Liu, C.; Hamzehpoor, E.; Sakai- Otsuka, Y.; Jadhav, T.; Perepichka, D. F. Angew. Chem. Int. Ed. 2020, 59, 23030–23034.
- 3(a) Huang, Y.; Egap, E. Open-Shell Organic Semiconductors: an Emerging Class of Materials with Novel Properties. Polym. J. 2018, 50, 603–614; (b) Imran, M.; Wehrmann, C. M.; Chen, M. S. Open-Shell Effects on Optoelectronic Properties: Antiambipolar Charge Transport and Anti-Kasha Doublet Emission from a N-Substituted Bisphenalenyl. J. Am. Chem. Soc. 2020, 142, 38–43.
- 4(a) Beldjoudi, Y.; Nascimento, M. A.; Cho, Y. J.; Yu, H.; Aziz, H.; Tonouchi, D.; Eguchi, K.; Matsushita, M. M.; Awaga, K.; Osorio-Roman, I.; Constantinides, C. P.; Rawson, J. M. Multifunctional Dithiadiazolyl Radicals: Fluorescence, Electroluminescence, and Photoconducting Behavior in Pyren-1-yl-dithiadiazolyl. J. Am. Chem. Soc. 2018, 140, 6260–6270; (b) Beldjoudi, Y.; Arauzo, A.; Campo, J.; Gavey, E. L.; Pilkington, M.; Nascimento, M. A.; Rawson, J. M. Structural, Magnetic, and Optical Studies of the Polymorphic 9’-Anthracenyl Dithiadiazolyl Radical. J. Am. Chem. Soc. 2019, 141, 6875–6889.
- 5(a) Su, Y.; Kinjo, R. Boron-Containing Radical Species. Coord. Chem. Rev. 2017, 352, 346–378;
(b) Power, P. P. Persistent and Stable Radicals of the Heavier Main Group Elements and Related Species. Chem. Rev. 2003, 103, 789–810;
(c) Kaim, W.; Hosmane, N. S.; Záliš, S.; Maguire, J. A.; Lipscomb, W. N. Boron Atoms as Spin Carriers in Two- and Three-Dimensional Systems. Angew. Chem. Int. Ed. 2009, 48, 5082–5091;
(d) Hicks; Robin, G. Stable Radicals, John Wiley & Sons, Ltd., United Kingdom, 2010;
(e) Renaud, P. Boron in Radical Chemistry, John Wiley & Sons, Ltd., United Kingdom, 2012;
10.1002/9781119953678.rad020 Google Scholar(f) Chivers, T.; Konu, J. Stable and Persistent Radicals of Group 13-17 Elements. In Comprehensive Inorganic Chemistry II (Second Edition), 2013, Volume 1, Elsevier, Amsterdam, pp. 349–373.
- 6(a) Hinchliffe, A.; Mair, F. S.; McInnes, E. J. L.; Pritchard, R. G.; Warren, J. E. Light Group 13 Chloride Diazadiene Complexes: Consequences of Varying Substituent Bulk. Dalton Trans. 2008, 222–233; (b) Wood, T. K.; Piers, W. E.; Keay, B. A.; Parvez, M. Spirocyclic Boronium Ions: Precursors to Persistent Neutral Radicals. Chem. Commun. 2009, 5147–5149; (c) Mansell, S. M.; Adams, C. J.; Bramham, G.; Haddow, M. F.; Kaim, W.; Norman, N. C.; McGrady, J. E.; Russell, C. A.; Udeen, S. J. Synthesis and Characterisation of the Persistent Radical [BCl2(bipy)]˙. Chem. Commun. 2010, 5070–5072; (d) Aramaki, Y.; Omiya, H.; Yamashita, M.; Nakabayashi, K.; Ohkoshi, S.; Nozaki, K. Synthesis and Characterization of B-Heterocyclic π-Radical and Its Reactivity as a Boryl Radical. J. Am. Chem. Soc. 2012, 134, 19989–19992; (e) Fedushkin, I. L.; Markina, O. V.; Lukoyanov, A. N.; Morozov, A. G.; Baranov, E. V.; Maslov, M. O.; Ketkov, S. Y. Boron Complexes of Redox-Active Diimine Ligand. Dalton Trans. 2013, 7952–7961; (f) Bamford, K. L.; Longobardi, L. E.; Liu, L.; Grimme, S.; Stephan, D. W. FLP Reduction and Hydroboration of Phenanthrene o-Iminoquinones and α-Diimines. Dalton Trans. 2017, 5308–5319.
- 7(a) Pal, S. K.; Itkis, M. E.; Tham, F. S.; Reed, R. W.; Oakley, R. T. Resonating Valence-Bond Ground State in a Phenalenyl-Based Neutral Radical Conductor. Science 2005, 309, 281–284, and refs therein;
(b) Longobardi, L. E.; Liu, L.; Grimme, S.; Stephan, D. W. Stable Borocyclic Radicals via Frustrated Lewis Pair Hydrogenations. J. Am. Chem. Soc. 2016, 138, 2500–2503;
(c) Longobardi, L. E.; Zatsepin, P.; Korol, R.; Liu, L.; Grimme, S.; Stephan, D. W. Reactions of Boron-Derived Radicals with Nucleophiles. J. Am. Chem. Soc. 2017, 139, 426–435;
(d) Robinson, G. H.; Wang, Y.; Xie, Y.; Wei, P.; Blair, S.; Cui, D.; Johnson, M. K.; Schaefer, H. F. Stable Boron Dithiolene Radicals. Angew. Chem. Int. Ed. 2018, 130, 7991–7994.
10.1002/ange.201804298 Google Scholar
- 8 Liu, L. L.; Stephan, D. W. Radicals Derived from Lewis Acid/base Pairs. Chem. Soc. Rev. 2019, 48, 3454–3463.
- 9 Bleaney, B.; Bowers, K. D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. London, Ser. A 1952, 214, 451–465.
- 10 Tan, G.; Wang, X. Isolable Radical Ions of Main-Group Elements: Structures, Bonding and Properties. Chin. J. Chem. 2018, 36, 573–586.
- 11 Kasha, M. Characterization of Electronic Transitions in Complex Molecules. Discuss. Faraday Soc. 1950, 9, 14–19. Kasha's rule is a principle in the photochemistry of electronically excited molecules. The rule states that photon emission (fluorescence or phosphorescence) occurs in appreciable yield only from the lowest excited state of a given multiplicity.
- 12Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lippa-rini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16 Rev. C.01, Wallingford, CT, 2016.
- 13 Pople, J. A.; Gill, P. M.; Handy, N. C. Spin-Unrestricted Character of Kohn-Sham Orbitals for Open-Shell Systems. Int. J. Quantum Chem. 1995, 56, 303–305.
- 14 Laurent, A. D.; Jacquemin, D. TD-DFT Benchmarks: a Review. Int. J. Quantum Chem. 2013, 113, 2019–2039.
- 15 Guo, H.; Jing, Y.; Yuan, X.; Ji, S.; Zhao, J.; Li, X.; Kan, Y. Highly Selective Fluorescent OFF-ON Thiol Probes Based on Dyads of BODIPY and Potent Intramolecular Electron Sink 2,4-dinitrobenzenesulfonyl Subunits. Org. Biomol. Chem. 2011, 9, 3844–3853.
- 16 Shafikov, M. Z.; Brandl, F.; Dick, B.; Czerwieniec, R. Can Coumarins Break Kasha's Rule? J. Phys. Chem. Lett. 2019, 10, 6468–6471.
- 17 Trosien, S.; Waldvogel, S. R. Synthesis of Highly Functionalized 9,10-Phenanthrenequinones by Oxidative Coupling Using MoCl5. Org. Lett. 2012, 14, 2976–2979.
- 18 Parks, D. J.; Piers, W. E.; Yap, G. P. A. Synthesis, Properties, and Hydroboration Activity of the Highly Electrophilic Borane Bis(pentafluorophenyl)borane, HB(C6F5)2. Organometallics 1998, 17, 5492–5503.




