Divergent Syntheses of Pyridoacridine Alkaloids via Palladium-Catalyzed Reductive Cyclization with Nitro-Biarenes
Bo Liu
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
‡ The authors contributed equally to this work.
Search for more papers by this authorShuping Wang
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
‡ The authors contributed equally to this work.
Search for more papers by this authorChanghao Bian
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorCorresponding Author
Hongze Liao
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hou-Wen Lin
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorBo Liu
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
‡ The authors contributed equally to this work.
Search for more papers by this authorShuping Wang
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
‡ The authors contributed equally to this work.
Search for more papers by this authorChanghao Bian
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorCorresponding Author
Hongze Liao
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hou-Wen Lin
Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
A divergent and novel protocol for the preparation of both pyrido[2,3,4-kl]acridine and pyrido[4,3,2-kl]acridine alkaloids was developed. This method featured the remote palladium-catalyzed reductive cyclization with Mo(CO)6 as reductant. A wide range of substrates including three types of nitro arenes were tolerated and afforded corresponding products in good to excellent yields. This method has been successfully applied to the total synthesis of norsegoline, styelsamine C and the skeleton of necatorone.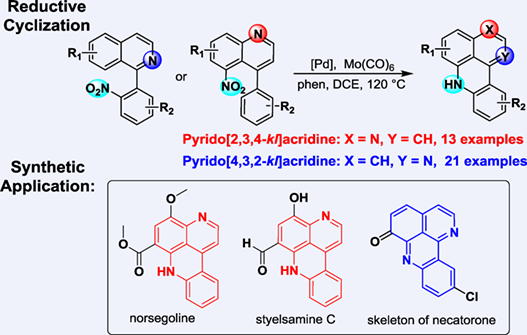
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100094-sup-0001-Supinfo.pdfPDF document, 11.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Joule, J. A.; Alvarez, M. Pyridoacridines in the 21st Century. Eur. J. Org. Chem. 2019, 2019, 5043–5072; (b) Plodek, A.; Bracher, F. New Perspectives in the Chemistry of Marine Pyridoacridine Alkaloids. Mar. Drugs 2016, 14, 26; (c) Delfourne, E.; Bastide, J. Marine Pyridoacridine Alkaloids and Synthetic Analogues as Antitumor Agents. Med. Res. Rev. 2003, 23, 234–252.
- 2(a) Ibrahim, S. R.; Mohamed, G. A. Marine Pyridoacridine Alkaloids: Biosynthesis and Biological Activities. Chem. Biodivers. 2016, 13, 37–47; (b) Sandjo, L. P.; Kuete, V.; Biavatti, M. W. Pyridoacridine alkaloids of marine origin: NMR and MS spectral data, synthesis, biosynthesis and biological activity. J. Org. Chem. 2015, 11, 1667–1699; (c) Molinski, T. F. Marine pyridoacridine alkaloids: structure, synthesis, and biological chemistry. Chem. Rev. 1993, 1825–1838.
- 3(a) Carstens, J.; Heinrich, M. R.; Steglich, W. Studies on the synthesis and biosynthesis of the fungal alkaloid necatorone. Tetrahedron Lett. 2013, 54, 5445–5447; (b) Heald, R. A.; Modi, C.; Cookson, J. C.; Hutchinson, I.; Laughton, C. A.; Gowan, S. M.; Kelland, L. R; Stevens M. F. G. Antitumor Polycyclic Acridines. 8.1 Synthesis and Telomerase-Inhibitory Activity of Methylated Pentacyclic Acridinium Salts. J. Med. Chem. 2002, 45, 590–597; (c) Klamann, J. D.; Fugmann, B.; Steglich, W. Alkaloidal Pigments from Lactarius Necator and L. Atroviridis. Phytochemisry 1989, 24, 3519–3522; (d) Fugmann, B.; Steffan, B.; Steglich, W. Necatorone, an Alkaloidal Pigment from the Gilled Toadstool Lactarius Necator (Agaricales). Tetrahedron Lett. 1984, 25, 3575–3578.
- 4(a) Borah, A.; Sharma, A.; Hazarika, H.; Sharma, K.; Gogoi, P. Synthesis of 1-Azaanthraquinone: Sequential C−N Bond Formation/Lewis Acid Catalyzed Intramolecular Cyclization Strategy. J. Org. Chem. 2017, 82, 8309–8316; (b) Yin, H.; Shan, N.; Wang, S.; Yao, Z. J. Total Synthesis of Ascididemin-Type Alkaloids Using Alkyne Building Blocks. J. Org. Chem. 2014, 79, 9748–9753; (c) Yin, H.; Kong, F.; Wang, S.; Yao, Z.-J. Assembly of pentacyclic pyrido[4,3,2-mn]acridin-8-ones via a domino reaction initiated by Au(I)-catalyzed 6-endo-dig cycloisomerization of N-propargylaminoquinones. Tetrahedron Lett. 2012, 53, 7078–7082; (d) Fei, N.; Yin, H.; Wang, S.; Wang, H.; Yao, Z. J. CuCl2-Promoted 6-endo-dig Chlorocyclization and Oxidative Aromatization Cascade: Efficient Construction of 1-Azaanthraquinones from N-Propargylaminoquinones. Org. Lett. 2011, 13, 4208–4211.
- 5(a) Melzer, B. C.; Plodek, A.; Bracher, F. Functionalization of 4-bromobenzo[c][2,7]naphthyridine via regioselective direct ring metalation. A novel approach to analogues of pyridoacridine alkaloids. Beilstein J. Org. Chem. 2019, 15, 2304–2310; (b) Plodek, A.; König, M.; Bracher, F. Synthesis of the Azaoxoaporphine Alkaloid Sampangine and Ascididemin-Type Pyridoacridines through TMPMgCl·LiCl-Mediated Ring Closure. Eur. J. Org. Chem. 2015, 2015, 1302–1308; (c) Melzer, B.; Plodek, A.; Bracher, F. Total Synthesis of the Marine Pyridoacridine Alkaloid Demethyldeoxyamphimedine. J. Org. Chem. 2014, 79, 7239–7242; (d) Kristensen, J.; Petersen, I. Synthesis of pyridoacridines through anionic cascade ring closure. Synthesis 2014, 46, 1469–1474; (e) Petersen, I. N.; Crestey, F.; Kristensen, J. L. Total synthesis of ascididemin via anionic cascade ring closure. Chem. Commun. 2012, 48, 9092–9094.
- 6(a) Brahic, C.; Darro, F.; Belloir, M.; Bastide, J.; Kiss, R.; Delfourne, E. Synthesis and Cytotoxic Evaluation of Analogues of the Marine Pyridoacridine Amphimedine. Bioorg. Med. Chem. 2002, 10, 2845–2853; (b) Matsumoto, S. S.; Sidford, M. H.; Holden, J. A.; Barrows, L. R.; Copp, B. R. Mechanism of action studies of cytotoxic marine alkaloids: ascididemin exhibits thiol-dependent oxidative DNA cleavage. Tetrahedron Lett. 2000, 41, 1667–1670; (c) Alvarez, M.; Feliu, L.; Ajana, W.; Joule, J. A.; Fernandez-Puentes, J. L. Synthesis of Ascididemine and an Isomer. Eur. J. Org. Chem. 2000, 2000, 849–855; (d) Cuerva, J. M.; Cárdenas, D. J.; Echavarren, A. M. New synthesis of pyridoacridines based on an intramolecular aza-Diels-Alder reaction followed by an unprecedented rearrangement. Chem. Commun. 1999, 1721–1722; (e) Echavarren, A. M.; Stille, J. K. Total Synthesis of Amphimedine. J. Am. Chem. Soc. 1988, 110, 4051–4053.
- 7(a) Nakahara, S.; Kubo, A. Total Synthesis of Styelsamine C, and Formal Synthesis of Norsegoline. Heterocycles 2005, 65, 1925–1929; (b) Nakahara, S.; Kubo, A. Total Synthesis of Styelsamine C, a Cytotoxic Fused Tetracyclic Aromatic Alkaloid. Heterocycles 2004, 35, 2017–2018; (c) Alajarín, M.; Vidal, A.; Ortín, M.-M.; Tovar, F. Intramolecular [4 + 2] Cycloaddition Reactions of Ketenimines: A New Synthesis of Benz[b]acridines. Synthesis 2002, 2002, 2393–2398; (d) Kitahara, Y.; Onikura, H.; Kubo, A. Total Synthesis of Norsegoline. Nat. Prod. Lett. 1993, 2, 159–162.
- 8(a) Khalil, I. M.; Barker, D.; Copp, B. R. Bioinspired Syntheses of the Pyridoacridine Marine Alkaloids Demethyldeoxyamphimedine, Deoxyamphimedine, and Amphimedine. J. Org. Chem. 2016, 81, 282–289; (b) Skyler, D.; Heathcock, C. H. A Simple Biomimetic Synthesis of Styelsamine B. Org. Lett. 2001, 3, 4323–4324; (c) Gellerman, G.; Rudi, A.; Kashman, Y. Two Step Biomimetic Total Synthesis of Eilatin. Tetrahedron Lett. 1993, 34, 1823–1830; (d) Molinski, T. F. Marine Pyridoacridine Alkaloids: Structure, Synthesis, and Biological Chemistry. Chem. Rev. 1993, 93, 1825–1838.
- 9(a) Usman, M.; Hu, X.-D.; Liu, W.-B. Recent Advances and Perspectives in the Synthesis and Applications of Tetrahydrocarbazol-4-ones. Chin. J. Chem. 2020, 38, 737–752; (b) Ferretti, F.; Ramadan, D. R.; Ragaini, F. Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488.
- 10(a) Shi, L; Wen, M; Li F. Palladium-Catalyzed Tandem Carbonylative Aza-Wacker-Type Cyclization of Nucleophile Tethered Alkene to Access Fused N-Heterocycles. Chin. J. Chem. 2020, 39, 317–322; (b) Ford, R. L.; Alt, I.; Jana, N.; Driver, T. G. Intramolecular Pd-Catalyzed Reductive Amination of Enolizable sp3-C−H Bonds. Org. Lett. 2019, 21, 8827–8831; (c) El-Atawy, M. A.; Ferretti, F.; Ragaini, F. Palladium- Catalyzed Intramolecular Cyclization of Nitroalkenes: Synthesis of Thienopyrroles. Eur. J. Org. Chem. 2017, 2017, 1902–1910; (d) Ferretti, F.; El-Atawy, M. A.; Muto, S.; Hagar, M.; Gallo, E.; Ragaini, F. Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of β-Nitrostyrenes with Carbon Monoxide as the Reductant. Eur. J. Org. Chem. 2015, 2015, 5712–5715; (e) Platon, M.; Amardeil, R.; Djakovitch, L.; Hierso, J. C. Progress in palladium-based catalytic systems for the sustainable synthesis of annulated heterocycles: a focus on indole backbones. Chem. Soc. Rev. 2012, 41, 3929–3968; (f) Stokes, B. J.; Driver, T. G. Transition Metal-Catalyzed Formation of N-Heterocycles via Aryl-or Vinyl C-H Bond Amination. Eur. J. Org. Chem. 2011, 2011, 4071–4088; (g) Hsieh, T. H. H.; Dong, V. M. Indole synthesis: palladium-catalyzed C–H bond amination via reduction of nitroalkenes with carbon monoxide. Tetrahedron 2009, 65, 3062–3068; (h) Davies, I. W.; Smitrovich, J. H.; Sidler, R.; Qu, C.; Gresham, V.; Bazaral, C. A highly active catalyst for the reductive cyclization of ortho-nitrostyrenes under mild conditions. Tetrahedron 2005, 61, 6425–6437.
- 11(a) Su, Z.; Liu, B.; Liao, H.; Lin, H.-W. Synthesis of N-Heterocycles by Reductive Cyclization of Nitroalkenes using Molybdenum Hexacarbonyl as Carbon Monoxide Surrogate. Eur. J. Org. Chem. 2020, 26, 4059–4066; (b) Gao, Y.; Yang, S.; Huo, Y.; Hu, X. Q. Recent Progress on Reductive Coupling of Nitroarenes by Using Organosilanes as Convenient Reductants. Adv. Synth. Catal. 2020, 362, 3971–3986; (c) Gao, Y.; Yang, F.; Sun, F.; Liu, L.; Liu, B.; Wang, S. P.; Cheng, C. W.; Liao, H.; Lin, H. W. Total Synthesis of Aaptamine, Demethyloxyaaptamine, and Their 3-Alkylamino Derivatives. Org. Lett. 2019, 21, 1430–1433; (d) Formenti, D.; Ferretti, F.; Ragaini, F. Synthesis of N-Heterocycles by Reductive Cyclization of Nitro Compounds Using Formate Esters as Carbon Monoxide Surrogates. ChemCatChem 2018, 10, 148–152; (e) Chen, L.-W.; Xie, J.-L.; Song, H.-J.; Liu, Y.-X.; Gu, Y.-C.; Wang, Q.-M. Pd-Catalyzed cycloisomerization/nucleophilic addition/ reduction: an efficient method for the synthesis of spiro-pseudoindoxyls containing N,N’-ketal. Org. Chem. Front. 2017, 4, 1731–1735; (f) Zhou, F.; Wang, D.-S.; Driver, T. G. Palladium-Catalyzed Formation of N-Heteroarenes from Nitroarenes Using Molybdenum Hexacarbonyl as the Source of Carbon Monoxide. Adv. Synth. Catal. 2015, 357, 3463–3468; (g) Jana, N.; Zhou, F.; Driver, T. G. Promoting Reductive Tandem Reactions of Nitrostyrenes with Mo(CO)6 and a Palladium Catalyst to Produce 3H-Indoles. J. Am. Chem. Soc. 2015, 137, 6738–6741.
- 12Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, revision A.02, Gaussian, Inc., Wallingford, CT, 2016.
- 13 Saleh, B.; Essa, A.; Al-Shawi, S.; Jalbout, A. Correlation analysis of the substituent electronic effects on the Mulliken charge. Resonance and field effects of substituents at para-substituted styrenyl fullerene. J. Mol. Struct.: THEOCHEM 2009, 909, 107–110.
- 14 Dunn, S. H.; McKillop, A. Synthesis of Norsegoline. J. Chem. Soc., Perkin Trans. 1 1993, 879–880.
- 15The skeleton of necatorone was identified by comparison of their MS and NMR data with the literature Ref. [3d].




