Enzymatic Synthesis of a Diastereomer of Neoabyssomicin Derivative Using the Diels-Alderase AbyU
Wenjuan Ding
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
College of Oceanology, University of Chinese Academy of Sciences, 19 Yuquan Road, Beijing, 100049 China
Search for more papers by this authorChangbiao Chi
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorXiaoyi Wei
Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong, 510650 China
Search for more papers by this authorChangli Sun
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
Search for more papers by this authorJiajia Tu
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Search for more papers by this authorMing Ma
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Qinglian Li
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianhua Ju
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
College of Oceanology, University of Chinese Academy of Sciences, 19 Yuquan Road, Beijing, 100049 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWenjuan Ding
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
College of Oceanology, University of Chinese Academy of Sciences, 19 Yuquan Road, Beijing, 100049 China
Search for more papers by this authorChangbiao Chi
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorXiaoyi Wei
Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong, 510650 China
Search for more papers by this authorChangli Sun
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
Search for more papers by this authorJiajia Tu
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Search for more papers by this authorMing Ma
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Qinglian Li
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianhua Ju
CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, 164 West Xingang Road, Guangzhou, Guangdong, 510301 China
College of Oceanology, University of Chinese Academy of Sciences, 19 Yuquan Road, Beijing, 100049 China
Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), No. 1119, Haibin Rd., Nansha District, Guangzhou, Guangdong, 510301 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
The enzyme AbyU catalyses a Diels-Alder (DA) reaction during abyssomicin C biosynthesis. In this study, AbyU is shown to convert the native substrate of another Diels-Alderase (DAase), AbmU, to a new abyssomicin derivative, abyssomicin 7. Abyssomicin 7 is a diastereomer of the AbmU-derived, abyssomicin 6. Using structural analyses and site-directed mutagenesis, we unveil the molecular basis for production of abyssomicin 7, instead of abyssomicin 6, within the AbyU active site.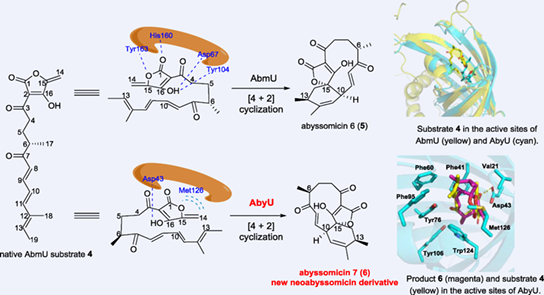
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100081-sup-0001-Supinfo.pdfPDF document, 2.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698.
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 2 Jeon, B. S.; Wang, S.-A.; Ruszczycky, M. W.; Liu, H. W. Natural [4 + 2]-Cyclases. Chem. Rev. 2017, 117, 5367–5388.
- 3 Byrne, M. J.; Lees, N. R.; Han, L.-C.; van der Kamp, M. W.; Mulholland, A. J.; Stach, J. E. M.; Willis, C. L.; Race, P. R. The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail. J. Am. Chem. Soc. 2016, 138, 6095–6098.
- 4 Li, Q.; Ding, W.; Tu, J.; Chi, C.; Huang, H.; Ji, X.; Yao, Z.; Ma, M.; Ju, J. Nonspecific heme-binding cyclase, AbmU, catalyzes [4 + 2] cycloaddition during neoabyssomicin biosynthesis. ACS Omega 2020, 5, 20548–20557.
- 5 Tian, Z.; Sun, P.; Yan, Y.; Wu, Z.; Zheng, Q.; Zhou, S.; Zhang, H.; Yu, F.; Jia, X.; Chen, D. An enzymatic [4 + 2] cyclization cascade creates the pentacyclic core of pyrroindomycins. Nat. Chem. Biol. 2015, 11, 259–265.
- 6 Zheng, Q.; Gong, Y.; Guo, Y.; Zhao, Z.; Wu, Z.; Zhou, Z.; Chen, D.; Pan, L.; Liu, W. Structural insights into a flavin-dependent [4 + 2] cyclase that catalyzes trans-decalin formation in pyrroindomycin biosynthesis. Cell Chem. Biol. 2018, 25, 718–727.
- 7 Tan, D.; Jamieson, C. S.; Ohashi, M.; Tang, M.-C.; Houk, K. N.; Tang, Y. Genome-mined Diels-Alderase catalyzes formation of the cis-octahydrodecalins of varicidin A and B. J. Am. Chem. Soc. 2019, 141, 769–773.
- 8 Zhang, Z.; Jamieson, C. S.; Zhao, Y. L.; Li, D. H.; Ohashi, M.; Houk, K. N.; Tang, Y. Enzyme-catalyzed inverse-electron demand Diels-Alder reaction in the biosynthesis of antifungal Ilicicolin H. J. Am. Chem. Soc. 2019, 141, 5659–5663.
- 9 Hantke, V.; Skellam, E. J.; Cox, R. J. Evidence for enzyme catalysed intramolecular [4 + 2] Diels-Alder cyclization during the biosynthesis of pyrichalasin H. Chem. Commun. 2020, 56, 2925–2928.
- 10 Gao, L.; Su, C.; Du, X.; Wang, R.; Chen, S.; Zhou, Y.; Liu, C.; Liu, X.; Tian, R.; Zhang, L.; Xie, K.; Chen, S.; Guo, Q.; Guo, L.; Hano, Y.; Shimazaki, M.; Minami, A.; Oikawa, H.; Huang, N.; Houk, K. N.; Huang, L.; Dai, J.; Lei, X. FAD-dependent enzyme-catalysed intermolecular [4 + 2] cycloaddition in natural product biosynthesis. Nat. Chem. 2020, 12, 620–628.
- 11 Ohashi, M.; Liu, F.; Hai, Y.; Chen, M. B.; Tang, M. C.; Yang, Z. Y.; Sato, M.; Watanabe, K.; Houk, K. N.; Tang, Y. SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis. Nature 2017, 549, 502–506.
- 12 Chen, Q.; Gao, J.; Jamieson, C.; Liu, J.; Ohashi, M.; Bai, J.; Yan, D.; Liu, B.; Che, Y.; Wang, Y.; Houk, K. N.; Hu, Y. Enzymatic intermolecular hetero- Diels-Alder reaction in the biosynthesis of tropolonic sesquiterpenes. J. Am. Chem. Soc. 2019, 141, 14052–14056.
- 13 Huang, H.; Song, Y.; Li, X.; Wang, X.; Ling, C.; Qin, X.; Zhou, Z.; Li, Q.; Wei, X.; Ju, J. Abyssomicin monomers and dimers from the marine-derived Streptomyces koyangensis SCSIO 5802. J. Nat. Prod. 2018, 81, 1892–1898.
- 14 Zhang, J. Y.; Li, B. X.; Qin, Y. J.; Karthik, L.; Zhu, G. L.; Hou, C. J.; Jiang, L.; Liu, M. M.; Ye, X.; Liu, M.; Hsiang, T.; Dai, H. Q.; Zhang, L. X.; Liu, X. T. A new abyssomicin polyketide with anti-influenza A virus activity from a marine-derived Verrucosispora sp. MS100137. Appl. Microbiol. Biot. 2020, 104, 1533–1543.
- 15 Bister, B.; Bischoff, D.; Ströbele, M.; Riedlinger, J.; Reicke, A.; Wolter, F.; Bull, A. T.; Zähner, H.; Fiedler, H. P.; Süssmuth, R. D. Abyssomicin C–A polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew. Chem. Int. Ed. 2004, 43, 2574–2576.
- 16 Song, Y.; Li, Q.; Qin, F.; Sun, C.; Liang, H.; Wei, X.; Wong, N.-K.; Ye, L.; Zhang, Y.; Shao, M.; Ju, J. Neoabyssomicins A–C, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802. Tetrahedron 2017, 73, 5366–5372.
- 17 Tu, J.; Li, S.; Chen, J.; Song, Y.; Fu, S.; Ju, J.; Li, Q. Neoabyssomicins A–C, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802. Microb. Cell Fact. 2018, 17, 28.
- 18 Li, Q.; Ding, W.; Yao, Z.; Tu, J.; Wang, L.; Huang, H.; Li, S.; Ju, J. AbmV catalyzes tandem ether installation and hydroxylation during neoabyssomicin/abyssomicin biosynthesis. Org. Lett. 2018, 20, 4854–4857.
- 19 Ji, X.; Tu, J.; Song, Y.; Zhang, C.; Wang, L.; Li, Q.; Ju, J. AbmV catalyzes tandem ether installation and hydroxylation during neoabyssomicin/ abyssomicin biosynthesis. ACS Catal. 2020, 10, 2591–2595.
- 20 Hong, B.; Phornphisutthimas, S.; Tilley, E.; Baumberg, S.; McDowall, K. J. Streptomycin production by Streptomyces griseus can be modulated by a mechanism not associated with change in the adpA component of the A-factor cascade. Biotechnol. Lett. 2007, 29, 57–64.
- 21 León, B.; Navarro, G.; Dickey, B. J.; Stepan, G.; Tsai, A.; Jones, G. S.; Morales, M. E.; Barnes, T.; Ahmadyar, S.; Tsiang, M. Abyssomicin 2 reactivates latent HIV-1 by a PKC-and HDAC-independent mechanism. Org. Lett. 2015, 17, 262–265.
- 22 Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. AutoDock4 and autoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791.
- 23 Cai, Y.; Hai, Y.; Ohashi, M.; Jamieson, C. S.; Garcia-Borras, M.; Houk, K. N.; Zhou, J.; Tang, Y. Structural basis for stereoselective dehydration and hydrogen-bonding catalysis by the SAM-dependent pericyclase LepI. Nat. Chem. 2019, 11, 812–820.
- 24 Dan, Q.; Newmister, S. A.; Klas, K. R.; Fraley, A. E.; McAfoos, T. J.; Somoza, A. D.; Sunderhaus, J. D.; Ye, Y.; Shende, V. V.; Yu, F.; Sanders, J. N.; Brown, W. C.; Zhao, L.; Paton, R. S.; Houk, K. N.; Smith, J. L.; Sherman, D. H.; Williams, R. M. Fungal indole alkaloid biogenesis through evolution of a bifunctional reductase/Diels-Alderase. Nat. Chem. 2019, 11, 972–980.
- 25 Zheng, Q.; Guo, Y.; Yang, L.; Zhao, Z.; Wu, Z.; Zhang, H.; Liu, J.; Cheng, X.; Wu, J.; Yang, H.; Jiang, H.; Pan, L.; Liu, W. Enzyme-dependent [4+2] cycloaddition depends on lid-like interaction of the N-terminal sequence with the catalytic core in PyrI4. Cell Chem. Biol. 2016, 23, 352–360.
- 26 Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Chirality 2013, 25, 243–249.
- 27 Wikler, M. A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Clinical and Laboratory Standards Institute, Wayne, PA, 2015, M07–A10.




